Chemical systems and industry: Identify and describe the impact of scientific knowledge of the hydrosphere on the quality of human, environmental, and socio-economic development
Unit 3: Treatment and purification of potable water
Emma Harrage
Unit 3 outcomes
By the end of this unit you will be able to:
- Classify water in terms of hardness.
- Explain water hardness and describe its effects.
- Describe the types of impurities found in water.
- Explain why water used by people and industries is purified.
- Define the water treatment and softening processes and give examples of them.
What you should know.
Before you start this unit, make sure you can:
- Recall the process of separating solids from liquids, as covered in Subject outcome 6.2, Unit 2.
- Understand that some compounds are soluble in water, as covered in Subject outcome 6.3, Unit 1.
- Understand the importance of the hydrosphere, as covered in Subject outcome 7.1, Unit 1.
- Understand that water becomes polluted with substances, as covered in Subject outcome 7.1, Unit 2.
Introduction
Before water is pumped to houses it goes through a treatment process which removes insoluble and some insoluble substances. Treating and purifying water is necessary for good health. Depending on the concentration of calcium and magnesium in the water, water can either be hard or soft. The hardness of water determines how much soap you will need when you are cleaning, and hard water causes a build-up of scale and scum which can affect the working of appliances.
Treatment and purification of potable water
When you drink a glass of water you are not just drinking water, but many other substances that are dissolved in the water. Some of these come from the process of making the water safe for humans to drink, while others come from the environment. Even if you took water from a mountain stream (which is often considered pure and bottled for people to consume), the water would still have impurities in it. Water pollution increases the amount of impurities in the water and sometimes makes the water unsafe for drinking. In this unit we will look at a few of the substances that make water impure and how we can make pure water. We will also look at the pH of water.
In Subject outcome 6.3 Unit 1: Principles of chemical reactions we saw how compounds can dissolve in water. Most of these compounds (e.g.[latex]\scriptsize \text{N}{{\text{a}}^{+}}\text{, C}{{\text{l}}^{-}}\text{, C}{{\text{a}}^{{2+}}},\text{ M}{{\text{g}}^{{2+}}}[/latex] etc.) are safe for humans to consume in the small amounts and are naturally present in water. It is only when the amounts of these ions rise above the safe levels that water is considered to be polluted.
You may have noticed sometimes that when you fill a glass with water straight from the tap, it has a sharp smell. This smell is the same smell that you notice around swimming pools and is from chlorine in the water. Chlorine is the most common compound added to water to make it safe for humans to use. Chlorine helps to remove bacteria and other biological contaminants in water. Other methods to purify water include filtration and ; a process of adding chemicals to water to help remove small particles.
The pH of water is also important. Water that is too basic (has a pH greater than 7) or too acidic (has a pH less than 7) may present problems when humans consume it. If you have ever noticed after swimming that your eyes are red or your skin is itchy, then the pH of the swimming pool was probably too basic or too acidic. This shows you just how sensitive we are to the smallest changes in our environment. The pH of water depends on what ions are dissolved in the water. Adding chlorine to water often lowers the pH.
Why and how water is purified
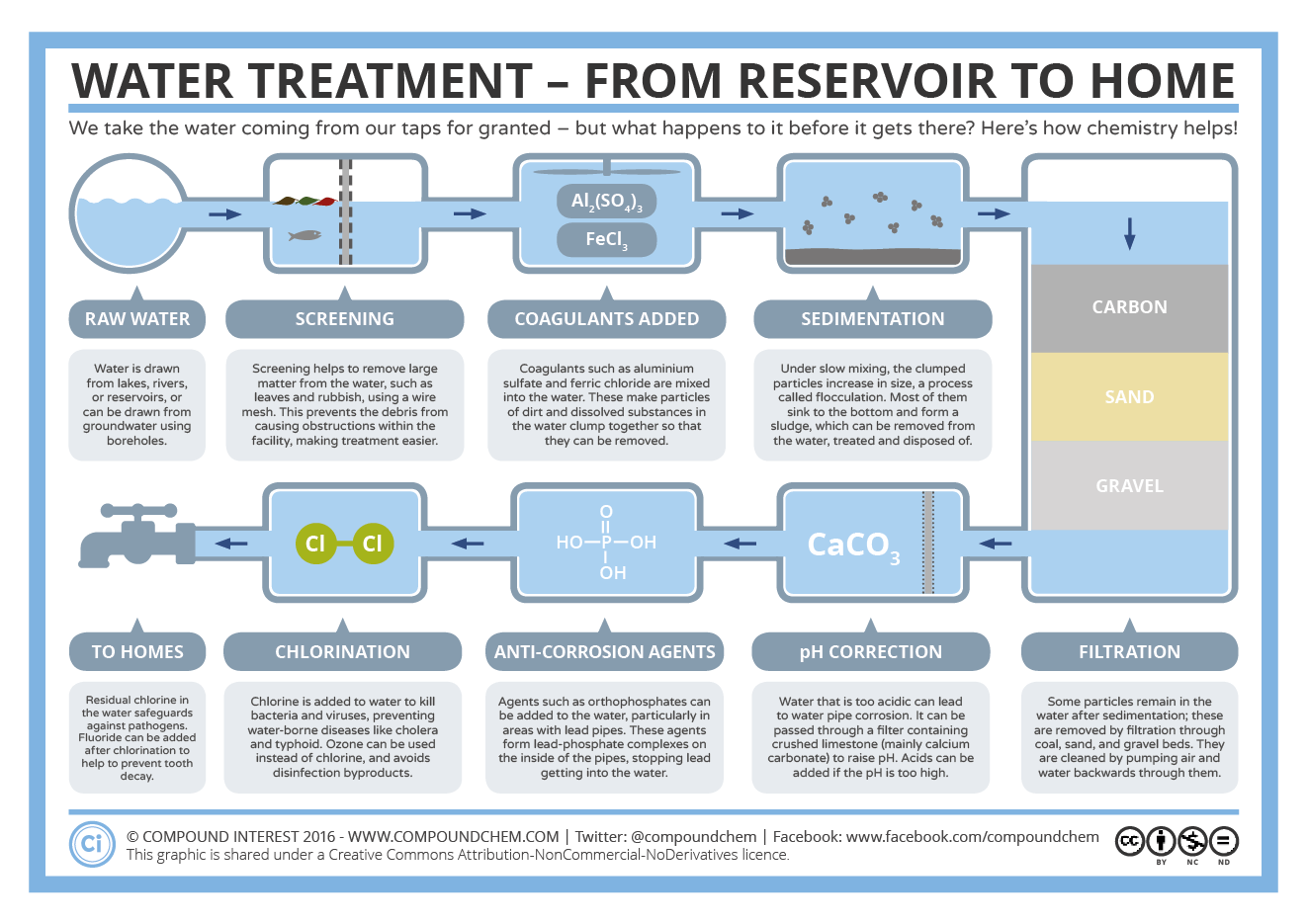
When water arrives at a purification plant or station it may contain both soluble and insoluble substances which may be harmful to human health. So before water comes out of our taps it goes through several processes:
- Screening: When raw water arrives at a purification station it passes through metal screens. These trap large living organisms (fish, crabs, floating plants, etc.), sticks, leaves and litter, but allow the water to pass through them.
- Coagulation: The raw water enters the middle of a spiral flocculator where slaked lime (calcium hydroxide) is added. This is thoroughly mixed in the rapidly moving water. The slaked lime attracts sand, silt and clay particles, some small living organisms, germs and all the ‘bad guys’ (pesticides, lead, mercury, arsenic, etc.) to form ‘clumps’.
- Flocculation: As the water begins to slow down in the outer section of the flocculator, the ‘clumps’ join to form ‘floc’.
- Sedimentation: The water then flows slowly into large sedimentation tanks. The ‘floc’ then settles to the bottom of the tank to form ‘sludge’. This is called sedimentation. The ‘sludge’ is sucked up by desludging bridges and sent to a sludge deposit site. The water in the top part of the tank is now cleaner. It flows over the side of the sedimentation tank into the carbonation tank.
- Carbonation: When water leaves the sedimentation tank it has a pH of about 10.5, from the slaked lime that was added. This high pH (alkaline) makes the water feel and taste soapy. In order to make the water less alkaline (have a lower pH), carbon dioxide is bubbled through the water. The pH of the water is now between 8.0 and 8.4. This makes the water taste and feel much better. At this pH level, calcium carbonate is deposited in the distribution pipes. This protects them from rusting.
- Filtration: The pH of the water has now been corrected through carbonation, but it still contains some small living organisms and germs. It flows into closed filter houses where it passes through sand filters. These are big, flat beds made up of different sized particles of sand and stone. As the water flows slowly through these filters all the small living organisms and some germs are trapped by the sand. The water now enters underground pipes.
- Chlorination: Even after the water has been filtered it still contains some germs. To kill these germs, chlorine gas (a disinfectant) is mixed with the water.
This clean water is pumped through underground pipes to booster pumping stations. The chlorine is only effective for 6 to 8 hours, so it is necessary to add chloramine (chlorine and ammonia) to prevent any other germs, which may get into the water, from growing or multiplying. From the booster pumping stations, the water is pumped into reservoirs. The water company then sells it to various local authorities that supply homes, schools, businesses, and factories with clean water.
Note
For a further explanation of water treatment, you can watch this edited video by Concerning Reality, How do water treatment plants work? (Duration: 8.01).
Water hardness
Water is classified as either soft or hard. contains relatively few minerals and lathers easily. is rich in minerals such as calcium and magnesium ions, which is the cause of “scale” in kettles. Water hardness is usually expressed as the number of parts per million (ppm) of calcium carbonate present in the water.
| Type of water | Hardness (ppm) |
| Soft water | [latex]\scriptsize 10-50[/latex] |
| Slightly hard water | [latex]\scriptsize 50-100[/latex] |
| Hard water | [latex]\scriptsize 100-200[/latex] |
| Very hard water | Over [latex]\scriptsize 200[/latex] |
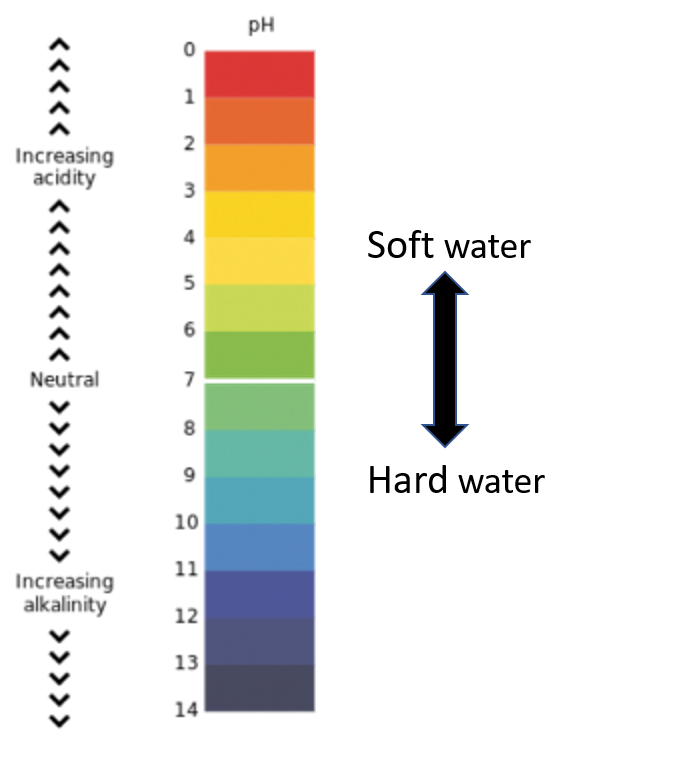
In general terms, areas with rainy climates have acidic water. Rain leaches out much of the mineral ions in the soil, replacing them with hydrogen ions. The result is that the water is rich in hydrogen ions and thus acidic (soft water). The reverse is the case in dry regions, where moisture evaporates, leaving the minerals intact. The result is water rich in minerals and thus alkaline (hard water).
Hard water has high mineral content because it is formed when water through the deposits of chalk and limestone which are made up of magnesium and calcium carbonates. It does not lather with soap, so it is not suitable for laundry purposes.
Kinds of hard water
There are three kinds of hard water:
- Temporary hardness: Temporary hardness is caused by soluble calcium bicarbonate [latex]\scriptsize \left( {\text{Ca}{{{\left( {\text{HC}{{\text{O}}_{2}}} \right)}}_{2}}} \right)[/latex] and/or magnesium bicarbonate [latex]\scriptsize \left( {\text{Mg}{{{\left( {\text{HC}{{\text{O}}_{3}}} \right)}}_{\text{2}}}} \right)[/latex] present in groundwater. Temporary hardness can be removed by boiling the water.
- Permanent hardness: When temporary hard water, caused by chlorides and sulphates of calcium and magnesium is heated, it forms calcium carbonate and/or magnesium carbonate [latex]\scriptsize \left( {\text{MgC}{{\text{O}}_{3}}} \right)[/latex] which precipitates out as scale or lime. Permanent hardness cannot be removed by boiling.
- Total hardness: Total hardness is the sum of all hardness constituents in water and is expressed as the equivalent concentration of calcium carbonate. It is primarily caused by calcium and magnesium but may include small amounts of metal ions such as iron.
Water in Gauteng in Rand Water’s area of supply ranges from [latex]\scriptsize 60\text{ to }110\text{ mg/lCaC}{{\text{O}}_{3}}[/latex], so Johannesburg has moderately soft to slightly hard water as does the rest of Gauteng. In Kwazulu-Natal and the Western Cape around Cape Town, which receives a large amount of rain, the water is generally soft.
Determining how hard the water is
Total water hardness (calcium and magnesium included) is expressed in parts per million (ppm) or milligram per litre (mg/l) of [latex]\scriptsize \text{CaC}{{\text{O}}_{3}}[/latex] (calcium carbonate). Water hardness tests usually measure the total concentration of Ca and Mg, the dominant divalent metal ions. In certain regions iron, aluminium and manganese can also be present in high concentrations. Water hardness can be determined by using test strips or hardness test kits, and more accurately by or ion specific .
The hardness of water is harmful to geysers and kettles as the deposition of salts occurs, which reduces the efficiency of the boiler. Hard water is safe to drink but using it for cleaning over a long period can lead to many problems. Hard water can cause linen and clothes to look dull and feel rough, it can cause ugly stains on white porcelain, scale build-up on taps, lower water pressure in showers due to clogged pipes, chalky or white residue or spots on dishes, and stains appearing in the shower. Under these circumstances water appliances have to work harder resulting in higher electricity bills.
Note
For a further explanation on the hardness of water you can watch this video by Fuse Schools: Hard & soft water (Duration: 4.21).
Softening water
Water hardness can be temporarily or permanently removed using various chemical or non-chemical methods.
The following are temporary chemical methods:
- Boiling: Temporary Hardness is due to bicarbonate ions, [latex]\scriptsize \text{HCO}_{3}^{-}[/latex], being present in the water. This type of hardness can be removed by boiling the water to remove the[latex]\scriptsize \text{C}{{\text{O}}_{\text{2}}}[/latex]. Soluble bicarbonates are converted into insoluble carbonates which are removed by filtration.
.
[latex]\scriptsize \text{Ca}{{\left( {\text{HC}{{\text{O}}_{\text{3}}}} \right)}_{\text{2}}}\text{ }\to \text{CaC}{{\text{O}}_{\text{3}}}\text{ + }{{\text{H}}_{\text{2}}}\text{O + C}{{\text{O}}_{\text{2}}}[/latex]
.
[latex]\scriptsize \text{Mg}{{\left( {\text{HC}{{\text{O}}_{\text{3}}}} \right)}_{\text{2}}}\to \text{MgC}{{\text{O}}_{\text{3}}}\text{+ }{{\text{H}}_{\text{2}}}\text{O + C}{{\text{O}}_{\text{2}}}[/latex] - Clark’s method: Clark’s method is a process for the large-scale removal of temporary hardness from water. Clark’s method involves adding a controlled quantity of slaked lime (calcium hydroxide). It removes the hardness of water by converting bicarbonates into carbonates. Slaked lime is itself a source of calcium ions (and hence hardness) so care must be taken to avoid adding too much.
.
[latex]\scriptsize \text{Ca}{{\left( {\text{OH}} \right)}_{\text{2}}}\text{ + Ca}{{\left( {\text{HC}{{\text{O}}_{\text{3}}}} \right)}_{\text{2}}}\text{ }\to \text{2CaC}{{\text{O}}_{\text{3}}}\text{ + 2}{{\text{H}}_{\text{2}}}\text{O}[/latex]
.
[latex]\scriptsize \text{Mg}{{\left( {\text{HC}{{\text{O}}_{\text{3}}}} \right)}_{\text{2}}}\text{+Ca}{{\left( {\text{OH}} \right)}_{\text{2}}}\to \text{CaC}{{\text{O}}_{\text{3}}}\text{ + MgC}{{\text{O}}_{\text{3}}}\text{ + 2}{{\text{H}}_{\text{2}}}\text{O}[/latex]
The following are permanent chemical methods:
- Washing soda method: In this method, water is treated with a calculated amount of washing soda, [latex]\scriptsize \text{N}{{\text{a}}_{\text{2}}}\text{C}{{\text{O}}_{\text{3}}}[/latex], which converts the chlorides and sulphates of calcium and magnesium into their respective carbonates, which get precipitated.
.
[latex]\scriptsize \begin{array}{*{20}{l}} {\text{CaC}{{\text{l}}_{\text{2}}}\text{ + N}{{\text{a}}_{\text{2}}}\text{C}{{\text{O}}_{\text{3}}}\text{ }\to \text{ CaC}{{\text{O}}_{\text{3}}}\text{ + 2NaCl}} \\ {\text{MgS}{{\text{O}}_{\text{4}}}\text{ + N}{{\text{a}}_{\text{2}}}\text{C}{{\text{O}}_{\text{3}}}\text{ }\to \text{MgC}{{\text{O}}_{\text{3}}}\text{ + N}{{\text{a}}_{\text{2}}}\text{S}{{\text{O}}_{\text{4}}}} \end{array}[/latex] - Ion exchange resin method: In this method, the permanent hardness of water is removed by using resins. [latex]\scriptsize \text{C}{{\text{a}}^{{2+}}}[/latex] and [latex]\scriptsize \text{M}{{\text{g}}^{{2+}}}[/latex] ions are exchanged with [latex]\scriptsize \text{C}{{\text{l}}^{\text{-}}}[/latex] and [latex]\scriptsize \text{SO}_{4}^{{2-}}[/latex]ions.
- Calgon’s process: In this method, sodium-hexa-meta-phosphate known as Calgon is used. The hardness in water is removed by the adsorption of [latex]\scriptsize \text{C}{{\text{a}}^{{2+}}}[/latex] and [latex]\scriptsize \text{M}{{\text{g}}^{{2+}}}[/latex] ions.
The following are the two most widely used non-chemical methods of permanent water softening.
- Distillation: [latex]\scriptsize \text{C}{{\text{a}}^{{2+}}}[/latex] and [latex]\scriptsize \text{M}{{\text{g}}^{{2+}}}[/latex] can be removed by water. Distillation is, however, very expensive in most cases. Rainwater is soft because it is naturally distilled during the water cycle of evaporation, condensation, and precipitation.
- Reverse : this method uses an applied pressure gradient across a to overcome osmotic pressure and remove water molecules from the solution with hardness ions. The membrane has pores large enough to let water molecules through, but the ions [latex]\scriptsize \text{C}{{\text{a}}^{{2+}}}[/latex] and [latex]\scriptsize \text{M}{{\text{g}}^{{2+}}}[/latex] will not fit through the pores. These membranes are a type of water filter and need to be cleaned or replaced regularly.
Summary
In this unit you have learnt the following:
- Water is treated to remove insoluble and soluble substances which may have polluted the water.
- The treatment process involves several stages including the addition of chlorine to kill any bacteria which may be in the water.
- Water can be classified as hard or soft depending on the concentration of calcium and/or magnesium ions present.
- Hard water needs to be treated to remove these ions. Hard water can cause a scale build up in appliances and soap to not lather.
Unit 3: Assessment
Suggested time to complete: 20 minutes
- What is the purpose of chlorine?
- make water smell clean
- disinfect
- create floc
- make water clear
- The cleaning process of putting water through several screens is called:
- coagulation
- sedimentation
- filtration
- aeration
- Water supplied to homes is treated by local councils in water treatment plants. Which one of the following is NOT one of the stages of this water treatment?
- settling
- boiling
- disinfection
- screening
- One of the treatments of water for domestic use is to add a flocculent such as aluminium sulphate to the water stored in large tanks. This helps small particles fall to the bottom of the tanks. This stage of the treatment is called:
- screening
- fluoridation
- settling
- filtration
- Hardness of water is due mainly to the presence of salts of:
- Potassium
- Chlorine
- Magnesium
- Boron
- Calcium hydroxide is also called:
- slaked lime
- quicklime
- hydrated lime
- dehydrated lime
- Coagulation is a slow stirring process that causes the gathering together of small particles into larger, settleable particles.
- True
- False
- What is the purpose of coagulation and flocculation?
- to control corrosion
- to filter out suspended particles
- to remove particulate impurities, especially non-settleable solids, and colour from the water being treated
- to settle out larger suspended particles
- What is the purpose of a water treatment plant?
- to modify source water
- to produce safe and pleasant drinking water
- to provide work experience for operators
- to treat drinking water
- What is the purpose of filtration?
- to control corrosion
- to filter out suspended particles
- to gather together fine, light particles to form larger particles (floc) to aid the sedimentation and filtration processes
- to settle out larger suspended particles
- Sedimentation is a physical process used in wastewater treatment to:
- remove particles that are less dense than water.
- remove particles that are denser than water.
- remove material from the water.
- none of the above
- Which of the following chemicals is sometimes added in the process of coagulation and flocculation?
- aluminium sulphate
- aluminium oxide
- calcium chloride
- none of these
- Why are calcium and magnesium ions undesirable in water?
- They can be removed economically, and the mineral recovered.
- They cause hard water.
- They can cause undesirable colour in water.
- They can promote the growth of iron bacteria, which can cause bad tastes and odours.
- Water hardness is mainly as a result of:
- calcium ions
- magnesium ions
- multivalent cations
- a and b
- Water hardness cations attract and bind up with:
- natural skin oils.
- phosphate ions.
- soaps and detergents.
- all of the above
- When temporary hard water is boiled, one of the substances formed is:
- calcium bicarbonate
- calcium sulfate
- hydrogen chloride
- carbon dioxide
- Reverse osmosis is a process used to remove molecules through a:
- permeable membrane.
- non-permeable membrane.
- semipermeable membrane.
- The process does not use a membrane.
- Calgon is used for the removal of:
- sodium carbonate.
- permanent hardness of water.
- potassium carbonate.
- none of these
The full solutions are at the end of the unit.
Unit 3: Solutions
Unit 3: Assessment
- b
- c
- b
- c
- c
- a
- a
- d
- b
- b
- b
- a
- b
- d
- c
- d
- c
- b
Media Attributions
- Fig 1 © compound chem is licensed under a CC BY-NC (Attribution NonCommercial) license
- QR_Code_PSL2SO71U3_1
- Fig 2 © DHET is licensed under a CC BY (Attribution) license
- Fig 3 © Clipart best is licensed under a CC0 (Creative Commons Zero) license
- QR_Code_PSL2SO71U3_2
a process of adding chemicals to the water to help remove small particles
water which does not contain excess amounts of calcium or magnesium ions
water which has either calcium or magnesium ions
when a liquid moves slowly through a substance with very small holes in it
a process where a solution is added to another solution such that it reacts under conditions in which the added volume may be accurately measured
equipment used to measure the strength of light
a separation technique used to separate a soluble solid from a liquid, or to separate liquids which have different boiling points
the movement of molecules through a permeable membrane from an area of high concentration to an area of low concentration until the concentration is equal on both sides
is a membrane that only allows certain types of particles to move through it under certain conditions


