Matter and materials: Identify and describe atoms as the basic building block
Unit 2: Atomic number and atomic mass
Emma Harrage
Unit outcomes
By the end of this unit you will be able to:
- Tell the difference between atomic number and atomic mass and explain the difference between the two.
- Understand that each element has a different atomic mass and atomic number.
- Describe and identify an isotope.
- Identify common examples of isotopes.
What you should know
Before you start this unit, make sure you can:
- Identify the parts of an atom in Subject outcome 5.2, Unit 1: The atom.
Introduction
In this unit you will learn about atomic number, atomic mass, and isotopes. You will learn how each element has a different atomic mass and atomic number and how that relates to the charge of an atom.
You will learn that atoms of the same element can have a different number of neutrons and that these atoms are called isotopes. Isotopes are atoms that have the same number of protons, but they have a different number of neutrons.
Atomic number and mass
The chemical properties of an element are determined by the charge of its nucleus, so by the number of protons it has. This number is called the and is denoted by the letter [latex]\scriptsize Z[/latex].
The mass of an atom depends on how many nucleons its nucleus contains. The number of nucleons, which is the total number of protons plus neutrons, is called the atomic mass number and is denoted by the letter [latex]\scriptsize A[/latex].
Standard notation shows the chemical symbol, the atomic and the atomic number of an element as follows:
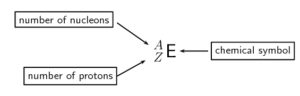
The nucleus of the element iron has [latex]\scriptsize 26[/latex] protons and [latex]\scriptsize 30[/latex] neutrons, so is denoted as: [latex]\scriptsize {}_{26}^{56}Fe[/latex] where the atomic number is [latex]\scriptsize Z = 26[/latex] and the mass number [latex]\scriptsize A = 56[/latex]. The number of neutrons is simply the difference [latex]\scriptsize N=A−Z=30[/latex].
Example 1
Use standard notation to represent sodium and give the number of protons, neutrons, and electrons in the element.
- Give the element symbol.
- Find the number of protons.
- Find the number of electrons.
- Find [latex]\scriptsize A[/latex].
- Work out the number of neutrons.
Solutions
- The element symbol for sodium is Na.
- Sodium has [latex]\scriptsize 11[/latex] protons, so we have: [latex]\scriptsize {}_{11}Na[/latex]
- Sodium is neutral, so it has the same number of electrons as protons. The number of electrons is [latex]\scriptsize 11[/latex].
- From the periodic table we see that [latex]\scriptsize A = 23[/latex].
- We know [latex]\scriptsize A[/latex] and [latex]\scriptsize Z[/latex] so we can find [latex]\scriptsize N: N = A − Z = 23 − 11 = 12[/latex]. In standard notation sodium is given by: [latex]\scriptsize {}_{11}^{23}Na[/latex].
The number of protons is [latex]\scriptsize 11[/latex], the number of neutrons is [latex]\scriptsize 12[/latex] and the number of electrons is [latex]\scriptsize 11[/latex].
Exercise 2.1
You will need a copy of the Periodic Table to complete these questions
- Copy the following table into your notebook and then complete the table:
Element Atomic Mass Atomic number Protons Electrons Neutrons Cu [latex]\scriptsize 12[/latex] Ni [latex]\scriptsize 28[/latex] [latex]\scriptsize 40[/latex] [latex]\scriptsize 20[/latex] - Use standard notation to represent the following elements:
- potassium
- copper
- chlorine
- For the element Cl, give the number of the following particles in the atom:
- protons
- neutrons
- electrons
- In each of the following cases, give the number or the element symbol represented by [latex]\scriptsize X[/latex].
- [latex]\scriptsize {}_{18}^{40}X[/latex]
- [latex]\scriptsize {}_{20}^{x}Ca[/latex]
- [latex]\scriptsize {}_{x}^{31}P[/latex]
- Which of the following atoms has [latex]\scriptsize 7[/latex] electrons?
- [latex]\scriptsize {}_{2}^{5}He[/latex]
- [latex]\scriptsize {}_{6}^{13}C[/latex]
- [latex]\scriptsize {}_{3}^{7}Li[/latex]
- [latex]\scriptsize {}_{7}^{15}N[/latex]
The full solutions are at the end of the unit.
Isotopes
The chemical properties of an element depend on the number of protons and electrons inside the atom. So, if a neutron or two is added or removed from the nucleus, then the chemical properties will not change. This means that such an atom would remain in the same place in the periodic table. For example, no matter how many neutrons we add or subtract from a nucleus with [latex]\scriptsize 6[/latex] protons, that element will always be called carbon and have the element symbol C (see the periodic table). Atoms that have the same number of protons (the same atomic number [latex]\scriptsize Z[/latex]), but a different number of neutrons (a different [latex]\scriptsize N[/latex] value and therefore a different mass number [latex]\scriptsize A[/latex]), are called .
The chemical properties of the different isotopes of an element are the same, but they might vary in how stable their nucleus is. We can also write elements as [latex]\scriptsize E–A[/latex], where the [latex]\scriptsize E[/latex] is the element symbol, and the [latex]\scriptsize A[/latex] is the atomic mass of that element. For example, Cl-35 has an atomic mass of 35 u ([latex]\scriptsize 17[/latex] protons and [latex]\scriptsize 18[/latex] neutrons), while Cl-37 has an atomic mass of 37 u ([latex]\scriptsize 17[/latex] protons and [latex]\scriptsize 20[/latex] neutrons).
In nature, the different isotopes occur in different percentages. For example, Cl-35 might make up [latex]\scriptsize 75 \%[/latex] of all chlorine atoms on Earth, and Cl-37 makes up the remaining [latex]\scriptsize 25 \%[/latex].
Activity 2.1: Building isotopes
Suggested time: 15 Minutes
What you need:
- internet access
What to do:
Go to this link.
- Click on ‘Isotopes and Atomic Mass’ and then select ‘Isotopes’. Change the number of neutrons in the hydrogen atom to make an isotope.
- Change the element to a different one and again change the number of neutrons to make a different isotope. Whilst you are adding neutrons, keep an eye on the pie chart as this will show you how abundant this isotope is in nature. Also note the stability of the isotope as you add neutrons.
- Go back to the main page and click on mixtures.
- Change the elements, select ‘Nature’s Mix’, and see the percentage of each type of isotope naturally found for that element.
What did you find?
Isotopes: You will have noted that as you added neutrons to the nucleus, the stability of the isotope changes as does its abundance in nature.

For example, [latex]\scriptsize \text{Carbon-13}[/latex] is a stable isotope, but as you add another neutron to make [latex]\scriptsize \text{Carbon-14}[/latex], the isotope becomes unstable.
Natures Mix: As you look at the different elements, you will see the isotopes which occur naturally in different colours, and on the right of the screen you will see the naturally occurring percentages of these isotopes.

For example, Magnesium has two isotopes which make up approximately [latex]\scriptsize 21 \%[/latex] of the composition of magnesium.
Exercise 2.2
- Atom A has [latex]\scriptsize 5[/latex] protons and [latex]\scriptsize 4[/latex] neutrons, and Atom B has [latex]\scriptsize 6[/latex] protons and [latex]\scriptsize 5[/latex] neutrons. These atoms are:
- allotropes
- isotopes
- isomers
- atoms of different elements
- For the sulphur isotopes [latex]\scriptsize {}_{16}^{32}S[/latex] and [latex]\scriptsize {}_{16}^{34}S[/latex], given the number of:
- protons
- nucleons
- electrons
- neutrons
The full solutions are at the end of the unit.
Note
If you need further explanations about isotopes watch this video: Fuse Schools: What are isotopes? (2.50).
Summary
In this unit you have learnt the following:
- The atomic number ([latex]\scriptsize Z[/latex]) is the number of protons in an atom.
- The atomic mass number ([latex]\scriptsize A[/latex]) is the number of protons and neutrons in the nucleus of an atom.
- The standard notation that is used to write an element, is: [latex]\scriptsize {}_{Z}^{A}E[/latex], where [latex]\scriptsize E[/latex] is the element symbol, [latex]\scriptsize A[/latex] is the atomic mass number and [latex]\scriptsize Z[/latex] is the atomic number.
- The isotope of a particular element is made up of atoms which have the same number of protons as the atoms in the original element, but a different number of neutrons. This means that not all atoms of an element will have the same atomic mass.
Unit 2: Assessment
Suggested time to complete: 20 minutes
- Give the standard notation for the following elements:
- beryllium
- [latex]\scriptsize \text{Carbon-12}[/latex]
- fluorine
- For each of the following elements give the number of protons, neutrons, and electrons in the element:
- [latex]\scriptsize {}_{78}^{195}Pt[/latex]
- [latex]\scriptsize {}_{53}^{127}I[/latex]
- Write down only the word/term for each of the following descriptions:
- The sum of the number of protons and neutrons in an atom.
- The defined space around an atom’s nucleus, where an electron is most likely to be found.
- The charge of an atom is:
- positive
- neutral
- negative
- none of the above.
- Using your periodic table, copy and complete the table below:
Isotope [latex]\scriptsize Z[/latex] [latex]\scriptsize A[/latex] Protons Electrons Neutrons [latex]\scriptsize \text{Carbon-14}[/latex] [latex]\scriptsize \text{Carbon-12}[/latex] [latex]\scriptsize \text{Iron-54}[/latex] [latex]\scriptsize \text{Iron-56}[/latex] [latex]\scriptsize \text{Iron-57}[/latex]
The full solutions are at the end of the unit.
Unit 2: Solutions
Exercise 2.1
- .
Element Atomic Mass Atomic number Protons Electrons Neutrons Cu [latex]\scriptsize 63.55[/latex] [latex]\scriptsize 29[/latex] [latex]\scriptsize 29[/latex] [latex]\scriptsize 29[/latex] [latex]\scriptsize 34[/latex] Ni [latex]\scriptsize 58.69[/latex] [latex]\scriptsize 28[/latex] [latex]\scriptsize 28[/latex] [latex]\scriptsize 28[/latex] [latex]\scriptsize 30[/latex] Ca [latex]\scriptsize 40[/latex] [latex]\scriptsize 20[/latex] [latex]\scriptsize 20[/latex] [latex]\scriptsize 20[/latex] [latex]\scriptsize 20[/latex] - .
- [latex]\scriptsize {}_{19}^{39}K[/latex]
- [latex]\scriptsize {}_{29}^{64}Cu[/latex]
- [latex]\scriptsize {}_{17}^{35}Cl[/latex]
- .
- [latex]\scriptsize 17[/latex]
- [latex]\scriptsize 18[/latex]
- [latex]\scriptsize 17[/latex]
- .
- [latex]\scriptsize X[/latex] = Ar
- [latex]\scriptsize x[/latex] = [latex]\scriptsize 40[/latex]
- [latex]\scriptsize x[/latex] = [latex]\scriptsize 15[/latex]
- d. Nitrogen
Exercise 2.2
- b. isotopes
- .
- [latex]\scriptsize 16[/latex]
- [latex]\scriptsize 1[/latex]
- [latex]\scriptsize 16[/latex]
- [latex]\scriptsize 16[/latex] and [latex]\scriptsize 18[/latex]
Unit 2: Assessment
- .
- [latex]\scriptsize {}_{4}^{9}B[/latex]
- [latex]\scriptsize {}_{6}^{12}C[/latex]
- [latex]\scriptsize {}_{9}^{19}F[/latex]
- .
- Pt: [latex]\scriptsize 78[/latex] electrons and protons = [latex]\scriptsize 78[/latex]; [latex]\scriptsize 117[/latex] neutrons
- I: [latex]\scriptsize 53[/latex] electrons and protons; [latex]\scriptsize 74[/latex] neutrons
- .
- Mass number
- Electron shell
- b. Neutral
- .
Isotope [latex]\scriptsize Z[/latex] [latex]\scriptsize A[/latex] Protons Electrons Neutrons [latex]\scriptsize \text{Carbon-14}[/latex] [latex]\scriptsize 6[/latex] [latex]\scriptsize 14[/latex] [latex]\scriptsize 6[/latex] [latex]\scriptsize 6[/latex] [latex]\scriptsize 8[/latex] [latex]\scriptsize \text{Carbon-12}[/latex] [latex]\scriptsize 6[/latex] [latex]\scriptsize 12[/latex] [latex]\scriptsize 6[/latex] [latex]\scriptsize 6[/latex] [latex]\scriptsize 6[/latex] [latex]\scriptsize \text{Iron-54}[/latex] [latex]\scriptsize 26[/latex] [latex]\scriptsize 54[/latex] [latex]\scriptsize 26[/latex] [latex]\scriptsize 26[/latex] [latex]\scriptsize 28[/latex] [latex]\scriptsize \text{Iron-56}[/latex] [latex]\scriptsize 26[/latex] [latex]\scriptsize 56[/latex] [latex]\scriptsize 26[/latex] [latex]\scriptsize 26[/latex] [latex]\scriptsize 20[/latex] [latex]\scriptsize \text{Iron-57}[/latex] [latex]\scriptsize 26[/latex] [latex]\scriptsize 57[/latex] [latex]\scriptsize 26[/latex] [latex]\scriptsize 26[/latex] [latex]\scriptsize 31[/latex]
Media Attributions
- Standard notation © Siyavula is licensed under a CC BY (Attribution) license
- Phet Carbon isotope © Phet is licensed under a CC BY (Attribution) license
- Phet isotopes mixture of magnesium © Phet is licensed under a CC BY (Attribution) license
the number of protons in an atom
the number of neutrons and protons in an atom
elements that have the same number of protons and electrons but a different number of neutrons in each atom
