Matter and materials: Identify, describe and apply properties of the periodic table
Unit 2: Electron configuration
Emma Harrage
Unit outcomes
By the end of this unit you will be able to:
- Arrange electrons into core and valence electrons and write electron configuration of first 20 elements.
- Write the electron configuration for the first 20 elements.
What you should know:
Before you start this unit, make sure you can:
- Confidently complete all the work in Subject outcome 5.2, Unit 2: Atomic number and atomic mass.
- Confidently complete all the work in Subject outcome 5.3, Unit 1: The periodic table.
Introduction
In this unit you will learn to arrange the first [latex]\scriptsize 20[/latex] elements into groups of core and valence electrons and write their electron configurations. The first [latex]\scriptsize 20[/latex] elements start at hydrogen (H) and continue vertically across the table until you reach calcium (Ca). They are called the first [latex]\scriptsize 20[/latex] elements because of their atomic number.
Electron configuration
As we learnt in SO 5.2 Unit 2: Atomic number and atomic mass, each element is made up of atoms which differ from each other because they have a different number of electrons, protons, and neutrons. The number of protons in a neutral atom of an element is called the atomic number.
If you look at the periodic table in Figure 1, you will see that there is a number above each element. Remember this is the atomic number. The number at hydrogen is [latex]\scriptsize 1[/latex], which means that it has [latex]\scriptsize 1[/latex] electron and [latex]\scriptsize 1[/latex] proton. If you look at copper (Cu), its atomic number is [latex]\scriptsize 29[/latex], which means that it has [latex]\scriptsize 29[/latex] electrons and [latex]\scriptsize 29[/latex] protons.
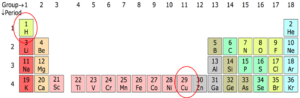
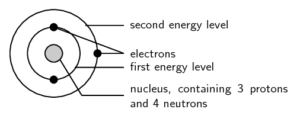
is the way electrons are arranged around the nucleus.
We start with a simple view of the arrangement or configuration of electrons around an atom. This view simply states that electrons are arranged in energy levels (or shells) around the nucleus of an atom. The period number of an electron tells us how many electron shells it has:
- hydrogen is in period so it will only have [latex]\scriptsize 1[/latex] electron shell.
- potassium is in period [latex]\scriptsize 4[/latex] so it will have [latex]\scriptsize 4[/latex] electron shells.
These energy levels are numbered 1, 2, 3, 4, etc.
The number of electrons an element has on its outer most shell is given by the group number for Groups [latex]\scriptsize 1[/latex] and [latex]\scriptsize 2[/latex],and is [latex]\scriptsize 10[/latex] minus the group number for Groups [latex]\scriptsize 13[/latex] to [latex]\scriptsize 17[/latex]. For example, calcium has [latex]\scriptsize 2[/latex] electrons because it is in Group [latex]\scriptsize 2[/latex] and oxygen, which is in Group [latex]\scriptsize 16[/latex], has [latex]\scriptsize 16[/latex]– [latex]\scriptsize 10=6[/latex]. This means it has [latex]\scriptsize 6[/latex] electrons on its outermost shell.
Core and valence electrons
Electrons in the outermost energy level of an atom are called . The electrons that are in the energy shells closer to the nucleus are called . Core electrons are all the electrons in an atom, excluding the valence electrons. An element that has its valence energy level full is more stable and less likely to react than other elements with a valence energy level that is not full.
Core electrons closest to the nucleus have the lowest energy. Electrons further away from the nucleus have a higher energy.
The Importance of electron configuration
During chemical reactions, when atoms come into contact with one another, it is the electrons of these atoms that will interact first. More specifically, it is the valence electrons of the atoms that will determine how they react with one another.
An atom is at its most stable and therefore unreactive when all its orbitals are full. An atom is least stable (and therefore most reactive) when its valence electron orbitals are not full.
The valence electrons are largely responsible for an element’s chemical behaviour and elements that have the same number of valence electrons often have similar chemical properties.
The most stable elements are the ones that have full energy levels. These configurations occur in Group [latex]\scriptsize 18[/latex] elements, otherwise known as the noble gases. The noble gases are very stable elements that do not react easily with any other elements. This is due to their full energy levels. All elements would like to reach the most , in other words, all elements want to be noble gases.
This principle of stability is sometimes referred to as the . An octet is a set of [latex]\scriptsize 8[/latex], and the number of electrons in a full energy level is [latex]\scriptsize 8[/latex].
Elements are placed in groups because of their electron configuration. Group [latex]\scriptsize 1[/latex] metals have [latex]\scriptsize 1[/latex] electron on their outer shell, Group [latex]\scriptsize 2[/latex] elements have [latex]\scriptsize 2[/latex] electrons on their outer shell which they will lose easily when bonding to become ions. Group [latex]\scriptsize 17[/latex] elements need one electron, Group [latex]\scriptsize 16[/latex] needs [latex]\scriptsize 2[/latex] electrons to have a full outer shell. The noble gases, Group [latex]\scriptsize 18[/latex], are very unreactive and stable because their shells are full.
Note
Here are some key points to remember:
Electron configuration diagrams are drawn as concentric circles around the nucleus and only show the electrons in each level. Electrons are usually drawn in pairs.
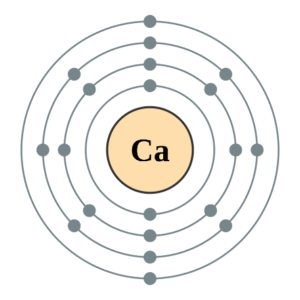
The first energy level can hold [latex]\scriptsize 2[/latex] electrons, the second energy level can hold [latex]\scriptsize 8[/latex] electrons, the third level can hold [latex]\scriptsize 8[/latex] and the fourth can hold [latex]\scriptsize 2[/latex]. So, it goes [latex]\scriptsize 2,8,8,2[/latex].
Elements want to have a stable electron configuration. They will either gain or lose valence electrons, so they can have either [latex]\scriptsize 2[/latex] or [latex]\scriptsize 8[/latex] electrons in their outer most energy shell.
Example 1
Lithium (Li) has an atomic number of [latex]\scriptsize 3[/latex], meaning that in a neutral atom, the number of electrons will also be [latex]\scriptsize 3[/latex]. Lithium has two energy levels; it is found in Group [latex]\scriptsize 2[/latex]. The first two electrons are found in the first energy level, while the third electron is found in the second energy level so, its electron configuration is [latex]\scriptsize 2,1[/latex].
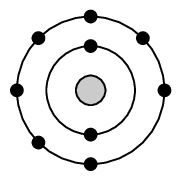
Fluorine is in period [latex]\scriptsize 2[/latex] so it will have two energy levels and in Group [latex]\scriptsize 17[/latex]. The first [latex]\scriptsize 2[/latex] electrons are found in the first energy level, while the other [latex]\scriptsize 7[/latex] are found in the second energy level.
This can be written as [latex]\scriptsize 2,7[/latex].
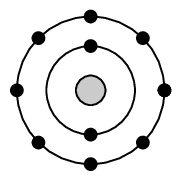
Neon is in period [latex]\scriptsize 2[/latex] which means that it has [latex]\scriptsize 2[/latex] energy levels, and [latex]\scriptsize 8[/latex] electrons on its outermost energy level. The first [latex]\scriptsize 2[/latex] electrons are found in the first energy level and the last [latex]\scriptsize 8[/latex] are found in the second energy level. This can be written as [latex]\scriptsize 2,8[/latex].
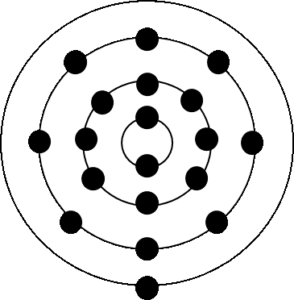
Calcium is in period [latex]\scriptsize 4[/latex] so it will have [latex]\scriptsize 4[/latex] energy levels and its electron configuration is [latex]\scriptsize 2,8,8,2[/latex].
Exercise 2.1
- Copy and complete the following table by writing the electron configuration for each element:
Element Symbol Atomic Number Electron Configuration Beryllium [latex]\scriptsize 2,6[/latex] C [latex]\scriptsize 6[/latex] [latex]\scriptsize 19[/latex] Ne Phosphorus - Draw shell diagrams showing the electron arrangement of the following elements:
- Nitrogen (N)
- Oxygen (O)
- Potassium (K)
The full solutions are at the end of the unit.
Summary
In this unit you have learnt the following:
- Electron configuration is the arrangement of electrons in energy shells.
- The electrons in the outermost energy level are called valence electrons.
- The electrons in an atom that are not valence electrons are called core electrons.
- Atoms with an outermost energy level that is full are less chemically reactive, and therefore more stable, than those atoms with an outermost energy level that is not full.
Unit 2: Assessment
Suggested time to complete: 30 minutes
- Copy the table below into your notebook and complete it:
Element Group Period Electron configuration Stable/unstable a. 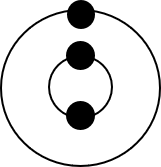
b. 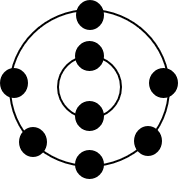
c. 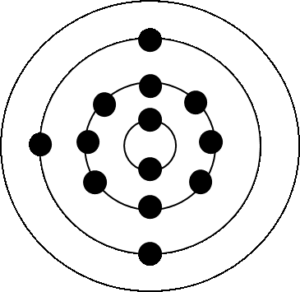
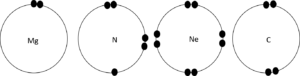
- Copy the table below into your notebook and complete it:
Element Number of energy levels Number of electrons Number of core electrons Number of valence electrons Mg N S C - Draw diagrams to show the valence electrons on the outer most energy level for the elements in Question 2.
The full solutions are at the end of the unit.
Unit 2: Solutions
Exercise 2.1
- .
Element Symbol Atomic Number Electron Configuration Beryllium Be [latex]\scriptsize 4[/latex] [latex]\scriptsize 2,2[/latex] Oxygen O [latex]\scriptsize 8[/latex] [latex]\scriptsize 2,6[/latex] Carbon C [latex]\scriptsize 6[/latex] [latex]\scriptsize 2,4[/latex] Potassium K [latex]\scriptsize 19[/latex] [latex]\scriptsize 2,8,8,1[/latex] Neon Ne [latex]\scriptsize 10[/latex] [latex]\scriptsize 2,8[/latex] Phosphorus P [latex]\scriptsize 15[/latex] [latex]\scriptsize 2,8,5[/latex] - .
- Nitrogen
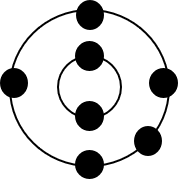
- Oxygen

- Potassium
- Nitrogen
Unit 2: Assessment
- .
Element Group Period Electron configuration Stable/unstable Lithium [latex]\scriptsize 1[/latex] [latex]\scriptsize 2[/latex] [latex]\scriptsize 2,1[/latex] unstable Oxygen [latex]\scriptsize 16[/latex] [latex]\scriptsize 2[/latex] [latex]\scriptsize 2,6[/latex] unstable Aluminium [latex]\scriptsize 13[/latex] [latex]\scriptsize 3[/latex] [latex]\scriptsize 2,8,3[/latex] unstable - .
Element Number of energy levels Number of electrons Number of core electrons Number of valence electrons Mg [latex]\scriptsize 3[/latex] [latex]\scriptsize 12[/latex] [latex]\scriptsize 10[/latex] [latex]\scriptsize 2[/latex] N [latex]\scriptsize 2[/latex] [latex]\scriptsize 7[/latex] [latex]\scriptsize 2[/latex] [latex]\scriptsize 5[/latex] S [latex]\scriptsize 3[/latex] [latex]\scriptsize 16[/latex] [latex]\scriptsize 10[/latex] [latex]\scriptsize 6[/latex] C [latex]\scriptsize 2[/latex] [latex]\scriptsize 6[/latex] [latex]\scriptsize 2[/latex] [latex]\scriptsize 4[/latex] - .
Media Attributions
- shortened periodic table © Pumbaa is licensed under a CC BY-SA (Attribution ShareAlike) license
- Shell diagram for lithium © Department of Higher Education is licensed under a CC BY (Attribution) license
- Flourine © Department of Higher Education and Training is licensed under a CC BY (Attribution) license
- Neon © Depratment of Higher Education and Training is licensed under a CC BY (Attribution) license
- Calcium © Pumbaa is licensed under a CC BY-SA (Attribution ShareAlike) license
- Lithium © Department of Higher Education and Training is licensed under a CC BY (Attribution) license
- oxygen © Department of Higher Education is licensed under a CC BY (Attribution) license
- Aluminium © Depratment of Higher Education and Training is licensed under a CC BY (Attribution) license
- Nitrogen © Department of Higher Education and Training is licensed under a CC BY (Attribution) license
- Potassium © Department of Higher Education is licensed under a CC BY (Attribution) license
- Shell diagrams solutions for assessment © Department of Higher Education is licensed under a CC BY (Attribution) license
the arrangement of electrons in an atom
the electrons on the outermost energy level.
electrons that are not valence electrons.
atoms will seek to lose or gain electrons to fill their outermost energy level.
need for electrons to have a full outer shell of electrons, which is 8.
