Chemical change: Differentiate between physical and chemical change
Unit 1: Chemical and physical change
Emma Harrage
Unit outcomes
By the end of this unit you will be able to:
- Identify and distinguish between both a chemical change and a physical change.
- Give examples of both.
- Understand conservation of mass.
- Write chemical equations.
What you should know:
Before you start this unit, make sure you can:
Confidently complete all the work in Subject outcome 5.4 Unit 1: Particles.
Confidently complete all the work in Subject outcome 5.4 Unit 2: Elements, mixtures and compounds.
Introduction
In this unit you will learn about chemical and physical changes and understand examples of both. A chemical change occurs because of a chemical reaction. The new substances formed are not easily separated into the elements from which they are composed. A physical change is a change of state, such as between solid, liquid or gas, and is easily reversed.
Physical changes
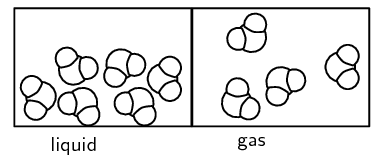
A is a change in which the particles of the substances that are involved do not change in any way. When water is heated, for example, both the temperature and energy of the water molecules increase, and the liquid water evaporates to form water vapour. When this happens, change has taken place, but the molecular structure of the water has not changed. This is an example of a physical change. All changes in state are physical changes.
[latex]\scriptsize {{\text{H}}_{\text{2}}}{{\text{O}}_{(l)}}\to {{\text{H}}_{\text{2}}}{{\text{O}}_{(g)}}[/latex]
Conduction (the transfer of energy through a material) is another example of a physical change. As energy is transferred from one material to another, the energy of each material is changed, but not its chemical makeup.
Dissolving one substance in another is also a physical change.
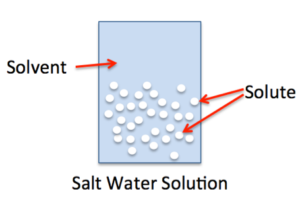
There are some important things to remember about physical changes in matter:
- Arrangement of particles
When a physical change occurs, the particles of the substance may re-arrange themselves, but the bonds between the atoms in the particles will not break. For example, when liquid water boils, the molecules will move apart but the molecule itself will stay intact. In other words, water will not break up into hydrogen and oxygen atoms.
- Conservation of mass
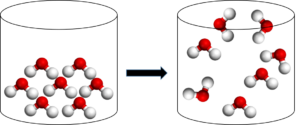
In a physical change the total mass, the number of atoms and the number of molecules will always stay the same. In other words, you will always have the same number of molecules or atoms at the end of the change as you had at the beginning.
- Energy changes
Energy changes may take place when there is a physical change in matter, but these energy changes are normally smaller than the energy changes that take place during a chemical change.
- Reversibility
Physical changes in matter are usually easier to reverse than chemical changes. Methods such as filtration and distillation can be used to reverse the change. Changing the temperature is another way to reverse a physical change. For example, a mixture of salt dissolved in water can be separated by filtration, and ice can be changed to liquid water and back again by changing the temperature.
Chemical changes
A is the result () of a reaction between two or more substances (). There are several ways chemical changes occur:
| Name of reaction | Reaction | General equation |
| Combination/Synthesis | Two or more elements combine to form one compound. | [latex]\scriptsize \text{A+B}\to \text{AB}[/latex] Example: [latex]\scriptsize \text{Sodium+Chlorine}\to \text{Sodium chloride}[/latex] [latex]\scriptsize \text{2Na+C}{{\text{l}}_{\text{2}}}\to \text{2NaCl}[/latex] |
| Decomposition | The opposite of a combination reaction. A complex molecule breaks down to make simpler ones. | [latex]\scriptsize \text{AB}\to \text{A+B}[/latex] Example: [latex]\scriptsize \text{Calcium carbonate}\to \text{Calcium oxide+Carbon dioxide}[/latex] [latex]\scriptsize \text{CaC}{{\text{O}}_{\text{3}}}\to \text{CaO+C}{{\text{O}}_{\text{2}}}[/latex] |
| Precipitation | Two solutions of soluble salts are mixed resulting in an insoluble solid (precipitate) forming. | [latex]\scriptsize \text{soluble saltA+ B}\to \text{precipitate + soluble salt C}[/latex] Example: [latex]\scriptsize \text{Sodium chloride+Silver nitrate}\to \text{Silver chloride+Sodium nitrate}[/latex] [latex]\scriptsize \text{NaCl+AgN}{{\text{O}}_{\text{3}}}\to \text{AgCl+NaN}{{\text{O}}_{\text{3}}}[/latex] |
| Neutralisation | An acid and a base react with each other. Generally, the product of this reaction is a salt and water. | Acid + Base → Salt + Water Example: [latex]\scriptsize \text{Hydrochloric acid+Sodium hydroxide}\to \text{Sodium chloride+water}[/latex] [latex]\scriptsize \text{HCl+NaOH}\to \text{NaCl+}{{\text{H}}_{\text{2}}}\text{O}[/latex] |
| Combustion | Oxygen combines with a compound to form carbon dioxide and water. These reactions are , meaning they give off heat. | [latex]\scriptsize \text{A+}{{\text{O}}_{\text{2}}}\to {{\text{H}}_{\text{2}}}\text{O+C}{{\text{O}}_{\text{2}}}[/latex] Example: [latex]\scriptsize \text{Glucose+oxygen Carbon dioxide+water}[/latex] [latex]\scriptsize {{\text{C}}_{\text{6}}}{{\text{H}}_{\text{12}}}{{\text{O}}_{\text{6}}}\text{+6}{{\text{O}}_{\text{2}}}\to 6\text{C}{{\text{O}}_{\text{2}}}\text{+6}{{\text{H}}_{\text{2}}}\text{O}[/latex] |
| Displacement | One element takes the place of another element in a compound. | [latex]\scriptsize \text{A+BC}\to \text{AC+B}[/latex] [latex]\scriptsize \text{Zinc+Copper sulphate}\to \text{Zinc sulphate+copper}[/latex] [latex]\scriptsize \text{Zn+CuS}{{\text{O}}_{\text{4}}}\to \text{ZnS}{{\text{O}}_{\text{4}}}\text{+Cu}[/latex] |

When a chemical change takes place, new substances are formed in a chemical reaction. These new products may have very different properties from the substances that were there at the start of the reaction. For example:
- Light emission – certain chemical reactions produce light because of the reaction.
- Bubbles – when a chemical change occurs in a liquid solution, and a gas is produced, bubbles can be seen in the liquid.
- Change of colour – a substance changing in colour is an excellent indication that some form of chemical change has happened. Colour changes are particularly noticeable in transition metals. An example of how a change in colour indicates a chemical change is when copper (which is an orange-brown colour) oxidises (reacts with oxygen) to produce copper oxide (which is a greenish colour).
- Odour change – chemical reactions can release molecules with certain smells, this is especially true of volatile chemicals.
- Change of temperature – chemical reactions necessitate a shift in the energy of an object, and this energy change is frequently detectable as a change in temperature.
- Formation of precipitate – precipitate refers to solid particles that are suspended in a solution and cause a clear solution to become cloudy.
- Irreversible changes – while physical changes can often be easily reversed, chemical changes are generally more difficult to reverse and seldom occur spontaneously in nature.
- Change in general form or composition – substantial changes in the composition or makeup of the substance often indicate chemical changes, such as when a wooden log turns into ash as it burns.
- Mass is conserved – the mass of a closed system of substances will remain constant, regardless of the processes acting inside the system. Matter can change form but cannot be created or destroyed. This is known as the and applies to physical and chemical changes.
Example 1
Explain the formation of iron sulfide.
Solution
Iron is a dull grey metal which is magnetic. Sulfur is a yellow non-metal which is not magnetic.
When iron and sulfur are mixed, they can easily be separated by using a magnet:

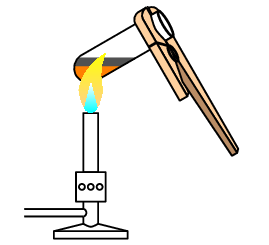
When the mixture is heated, the sulfur will change into an orange/brown liquid and give off a smell similar to rotting eggs. As heating continues the mixture will glow bright red and once sufficiently heated, the sulfur will react with the iron to form iron sulfide. Iron sulfide is a black solid which is not magnetic.

Example 2
Explain the of hydrogen peroxide.
Solution
The decomposition of hydrogen peroxide to form water and oxygen gas is an example of a chemical change.
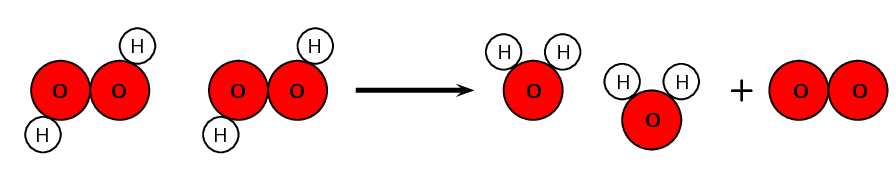
The chemical bonds between O and H in H2O2 are broken down, and new bonds between H and O are formed (to form H2O) and between O and O are formed (to form O2).
When this is done in a laboratory, a is used to speed up the reaction and during the reaction oxygen bubbles are released.
[latex]\scriptsize 2{{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}\to 2{{\text{H}}_{\text{2}}}\text{O + }{{\text{O}}_{2}}[/latex]
| Molecules | two molecules ([latex]\scriptsize 2{{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}[/latex]) | three molecules ([latex]\scriptsize \text{2}{{\text{H}}_{\text{2}}}\text{O+}{{\text{O}}_{\text{2}}}[/latex]) |
| Energy changes | energy taken in when bonds are broken | energy given off when new bonds are formed |
| Atoms are conserved | 4 oxygen atoms, 4 hydrogen atoms | 4 oxygen atoms, 4 hydrogen atoms |
You can watch a video of this reaction by jlambbio. (Duration: 1.56)
Activity 1: Make elephant toothpaste
What you need:
- ½ cup 6% hydrogen peroxide
- 1 teaspoon yeast
- 2 tablespoons hot water in a small dish
- 1 drop of food colouring
- washing-up liquid
- an small, empty, disposable plastic soda or water bottle
- a tray to stand the bottle on to catch the foam
- a funnel
What to do:
- Pour the hydrogen peroxide into the bottle.
- Mix the yeast in the water.
- Add the washing up liquid and food colouring to the hydrogen peroxide in the bottle.
- Add the yeast mixture to the bottle.
- Stand back and admire the reaction! This reaction is exothermic (it gives out heat), so do not hold the bottle or stand too close during the reaction.
- Record your observations.

What did you find:
When the yeast is added to the hydrogen peroxide, it foams rapidly and erupts out of the bottle. This is because hydrogen peroxide naturally breaks down over time into water and oxygen. Catalase, the enzyme in yeast, acts as a catalyst to speed up the reaction. The oxygen produced causes the washing up liquid to foam, and the food colouring just makes the whole thing look even cooler!
Note
Watch amazing chemical reactions. (Duration: 4.11)
Exercise 1.1
For each of the following, state whether a chemical or a physical change occurs.
- Melting candle wax.
- Frying an egg.
- Mixing hydrochloric acid (HCl) and magnesium ribbon (Mg) to form magnesium chloride (MgCl2).
- Dissolving salt in water.
- Melting ice cream.
The full solutions are at the end of the unit.
Note
For further explanation on chemical and physical changes watch this video by Chem Academy.
Physical vs. Chemical Changes – Explained (Duration: 7.40).
Representing chemical change by writing chemical equations
To represent chemical changes, we can write chemical equations.
For example, the reaction between iron and sulfur to form iron sulfide is:
[latex]\scriptsize \text{Fe+S}\to \text{FeS}[/latex]
The decomposition of hydrogen peroxide is:
[latex]\scriptsize 2{{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}\to 2{{\text{H}}_{\text{2}}}\text{O + }{{\text{O}}_{2}}[/latex]
In this equation you will notice that there is a 2 in front of the H2O2 and the H2O. This number represents the number of molecules of the substance in the reaction.
The subscript numbers on the H and O in the equation represents the number of atoms of the element in each molecule.
Summary
In this unit you have learnt the following:
- Matter does not stay the same. It may undergo physical or chemical changes.
- A physical change is a change that can be seen or felt, but that does not involve the breaking of bonds of the particles in the reaction. During a physical change, the form of matter may change, but not its identity.
- During a physical change, the arrangement of particles may change but the mass, number of atoms and number of molecules will stay the same.
- Physical changes involve small changes in energy and are easily reversible.
- A chemical change occurs when one or more substances change into other substances. A chemical reaction involves the formation of new substances with different properties. For example, hydrogen and oxygen react to form water.
- A chemical change may involve a decomposition or synthesis reaction. During a chemical change, the mass and number of atoms is conserved, but the number of molecules is not always the same.
Unit 1: Assessment
Suggested time to complete: 15 minutes
- Which of the following statements is incorrect for a chemical reaction?
- Heat may be given out but never absorbed.
- Sound may be produced.
- A colour change may take place.
- A gas may be given off.
- Which of the following is a physical change?
- Rusting of iron
- Combustion of magnesium ribbon
- Burning of candle
- Melting of wax
- Which of the following is a chemical change?
- Twinkling of stars
- Cooking of vegetables
- Cutting of fruit
- Boiling of water
- Sugar is dissolved into a mug of tea. What type of change is this?
- Chemical
- Physical
- Both chemical and physical
- Neither chemical nor physical
- For each of the following definitions give one word or term:
- A change that can be seen or felt, where the bonds in the particles involved are not broken.
- The formation of new substances in a chemical reaction.
- A reaction where a new product is formed from elements or compounds.
- For the following equations, state what kind of chemical change has occurred:
- [latex]\scriptsize \text{Magnesium + oxygen}\to \text{magnesium oxide}[/latex]
- [latex]\scriptsize \text{Hydrochloric acid + potassium hydroxide }\to \text{water + potassium chloride}[/latex]
- [latex]\scriptsize \text{Magnesium + copper sulphate }\to \text{copper + magnesium sulphate}[/latex]
The full solutions are at the end of the unit.
Unit 1: Solutions
Exercise 1.1
- Physical change – as soon as the wax cools down it will change back into a solid.
- Chemical change – the egg white has changed from a colourless liquid to a white solid.
- Chemical change – a new substance is formed. The mixture will give off bubbles (this is the hydrogen).
- Physical change – the salt can be separated from the water easily by evaporation.
- Physical change – the ice cream has changed state. It will be able to go back to being a solid when the temperature is reduced.
Unit 1: Assessment
- a
- d
- a
- b
- .
- physical change
- chemical change
- chemical change
- .
- synthesis/combination
- neutralisation
- displacement
Media Attributions
- dissolving salt © Libretext is licensed under a CC BY-NC (Attribution NonCommercial) license
- liquid to gas © Siyavula is licensed under a CC BY (Attribution) license
- liquid to gas 1 © Department of Higher Education and Training is licensed under a CC BY (Attribution) license
- Burning magnesium © Capt. John Yossarian is licensed under a CC BY-SA (Attribution ShareAlike) license
- Iron and sulfur © Libretext is licensed under a CC BY-NC (Attribution NonCommercial) license
- formation of iron sulfide © Siyavula is licensed under a CC BY (Attribution) license
- iron sulfide © Libretext is licensed under a CC BY-NC (Attribution NonCommercial) license
- Hydrogen peroxide © Siyavula is licensed under a CC BY (Attribution) license
- Elephant’s_toothpaste_experiment_with_food_coloring © Passavitch is licensed under a CC BY (Attribution) license
a change that does not involve the breaking of bonds within the particles of the reactants
the formation of new substances in a chemical reaction with a different chemical formula
the chemicals or result present at the end of the reaction
the chemicals at the start of the reaction
a reaction in which there is an overall release of energy (an increase in temperature of the surroundings)
the mass of a closed system of substances will remain constant, regardless of the processes acting inside the system; matter can change form but cannot be created or destroyed
breakdown
a substance that is used to speed up a chemical reaction
