Chemical change: Identify, describe, and apply principles of heat
Unit 1: First law of thermodynamics
Emma Harrage
Unit outcomes
By the end of this unit you will be able to:
- Understand the principle of the conservation of energy which is called the First Law of Thermodynamics.
- Understand that energy cannot be created or destroyed.
- Understand that the amount of energy in a closed system is constant.
What you should know
Before you start this unit, make sure you can:
- Explain what a force is. To revise this, go to Subject outcome 2.2, Unit 1.
- Define mechanical energy. To revise this, go to Subject outcome 2.3, Unit 1
Introduction
In this unit you learn about the principle of conservation of energy called the first law of thermodynamics. Energy cannot be created or destroyed; it can only be transferred from one form to another. This is the First Law of Thermodynamics and is about the conservation of energy in a system.
The first law of thermodynamics
This means that heat energy cannot be created or destroyed. It can, however, be transferred from one location to another and converted to and from other forms of energy.
Thermodynamics is taken from the Greek terms thermo meaning heat and dynamis meaning to force, so the word means heat force.
The change in the internal energy (i.e. the total energy) contained within a system is equal to the amount of heat supplied to that system, minus the amount of work performed by the system on its surroundings.
First law of thermodynamics equation
The first law of thermodynamics states that the change in internal energy of a system equals the net heat transfer into the system minus the net work done by the system. In equation form, the first law of thermodynamics is: [latex]\scriptsize \text{ }\!\!\Delta\!\!\text{ U = q + W}[/latex]
Where:
ΔU = change in internal energy of the system
q = algebraic sum of heat transfer between system and surroundings
W = work interaction of the system with its surroundings
For an isolated system, energy (E) always remains constant.
Work and heat are due to processes which add or subtract energy, they are not part of the system. While internal energy is a particular form of energy associated with the system; it’s a property of the system.
Thermodynamics often divides the universe into two categories: the system and its surroundings. In chemistry, the system almost always refers to a given chemical reaction and the container in which it takes place. In a closed system, the amount of energy in the system will remain constant.
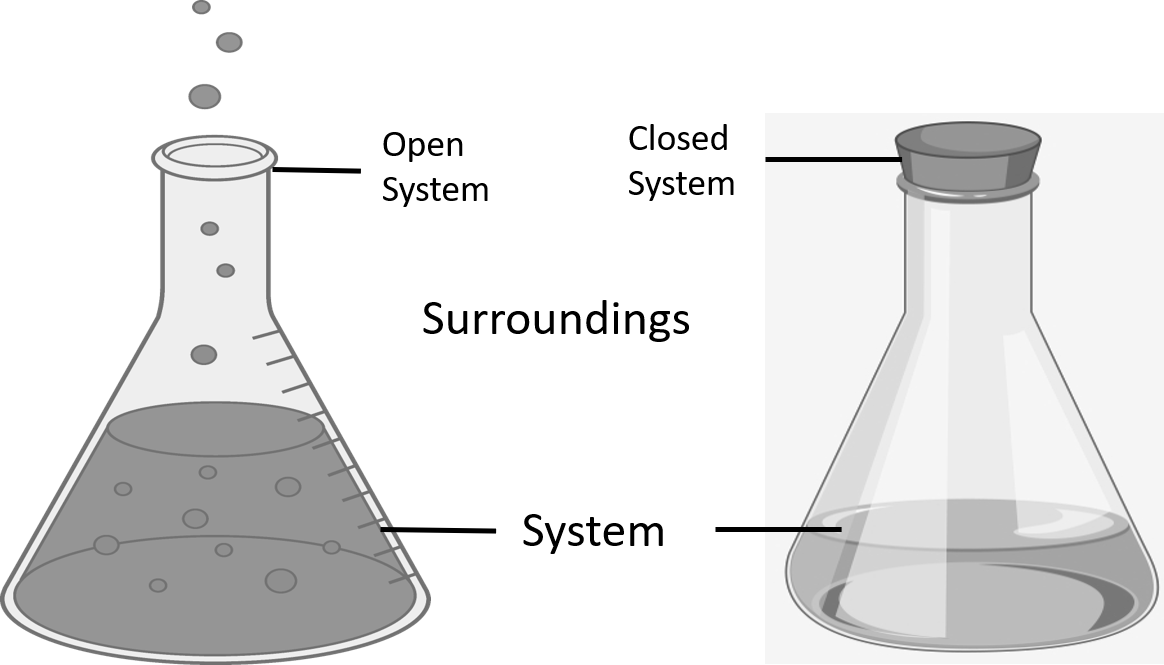
In chemical reactions, the energy that is absorbed in an chemical reaction must have been lost from the surroundings. Conversely, in an reaction, the heat that is released in the reaction is given off and absorbed by the surroundings.
Chemical reactions are often used to do work and exchange heat. For instance, when rocket fuel burns and causes a space shuttle to lift off from the ground, the chemical reaction, by propelling the rocket, is doing work by applying a force over a distance. When plants photosynthesise, they absorb heat energy from the sun to use as the energy needed to convert carbon dioxide and water into glucose and oxygen.
Heat, work, and internal energy
Heat transfer and doing work are the two everyday ways of bringing energy into or taking energy out of a system but the processes are quite different:
Heat transfer is driven by temperature differences.
Work involves a macroscopic force exerted through a distance.
The internal energy of a system is the sum of the kinetic and potential energies of its atoms and molecules. Kinetic plus potential energy is called mechanical energy. Because it is impossible to keep track of all individual atoms and molecules, we must deal with averages and distributions.
Whenever a system goes through any change due to the interaction of heat, work, and internal energy, it is followed by numerous energy transfers and conversions. However, during these transfers, there is no net change in the total energy.

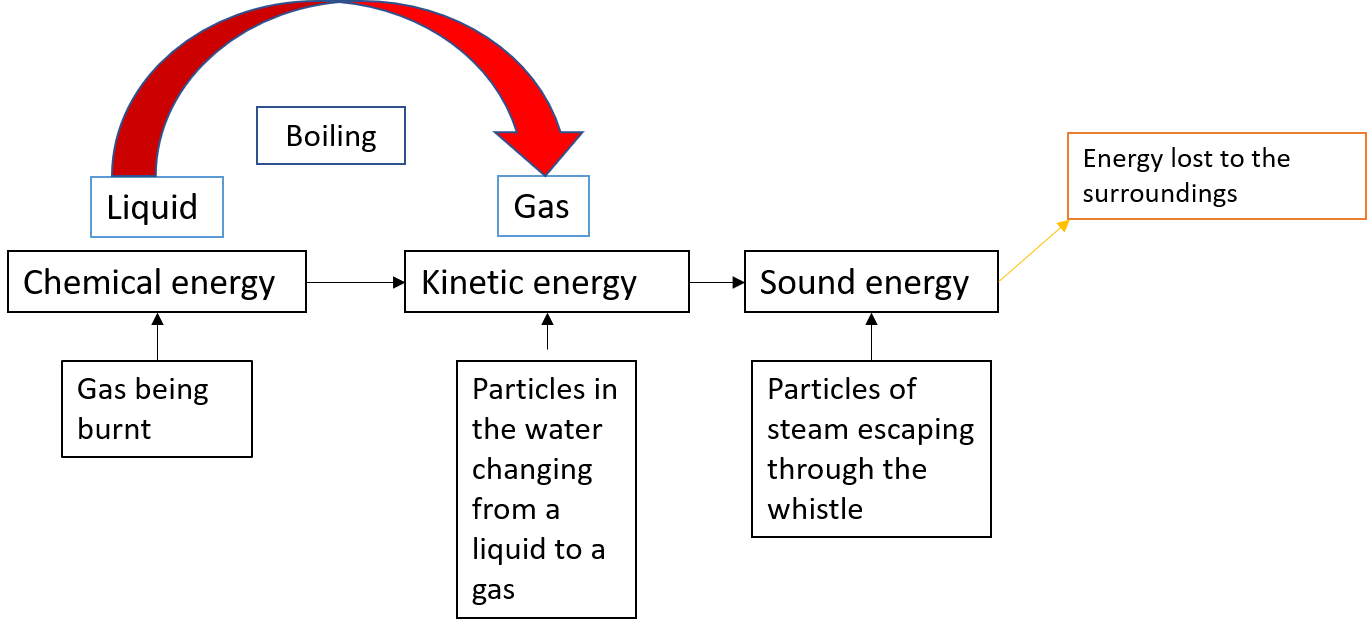
The water in the kettle in Figure 2 is turning to water vapour because heat is being transferred from the stove to the kettle. As the entire system gets hotter, work is done—from the evaporation of the water to the whistling of the kettle.
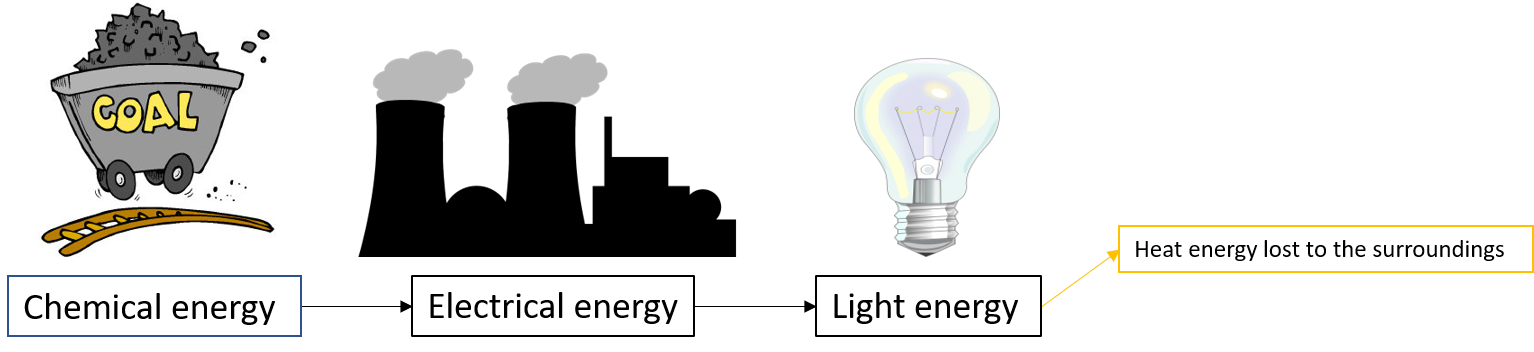
When you switch a light on the electrical energy needed comes from burning coal at a power station.
Ice needs to be maintained at a temperature below the freezing point of water to remain solid. On hot summer days, people often use ice to cool beverages. If you put ice in a glass of water and leave it, after a while the ice will have melted, but the temperature of the water will have decreased. This is because the ice has ‘pulled’ in the heat from the water. Interestingly, the ice and its melt water will stay at [latex]\scriptsize 0{{\text{ }}^{0}}\text{C}[/latex] until all of the ice has melted. The total amount of heat in the system has remained the same but has just moved towards a state where both the former ice cube (now water) and the water are the same temperature. As this is not a completely closed system, the water will eventually become warm again, as heat from the surroundings is transferred to the glass and its contents.
When you take a hot bath, the water initially feels very warm. As you soak heat is transferred from the water to you until your body feels like it is the same temperature as the water. Eventually, the water will cool down because heat is lost to the surroundings.
Plants perform one of the most biologically useful transformations of energy on Earth: they convert the energy of sunlight into the chemical energy stored within organic molecules.
Summary
In this unit you have learnt the following:
- First law of thermodynamics states that heat energy in a system is conserved.
- Energy cannot be created or destroyed, it can only be changed from one form to another.
- The thermodynamic equation is [latex]\scriptsize \text{ }\!\!\Delta\!\!\text{ U = q + W}[/latex], where
- U: internal energy – the sum of the kinetic and potential energies of a system’s atoms and molecules.
- q: heat – energy transferred because of a temperature difference
- W: work – energy transferred by a force moving through a distance.
- In a closed system, the amount of energy in the system will remain constant, but in an open system, energy will be lost to the surroundings.
Unit 1: Assessment
Suggested time to complete: 15 minutes
- Which of the following does NOT apply to the first law of thermodynamics?
- energy cannot be created
- energy cannot be destroyed
- energy cannot be transformed from one form to another
- the amount of energy within a closed system is constant
- Which of the following represents the first law of thermodynamics?
- While melting, an ice cube remains at the same temperature.
- The heat of an object explains how easily it changes temperature.
- After falling down the hill, a ball’s kinetic energy plus heat energy equals its potential energy.
- If a refrigerator is unplugged, eventually everything inside of it will return to room temperature.
- Explain and/or give definitions for the following:
- q (heat)
- W (work)
- a closed system
- internal energy
- Using the relevant terms from Question 3, briefly explain:
- Why your hot cup of tea will cool down if you leave it in a cool room for a period of time.
- On a cold winter evening, an ice-cold beer’s temperature will not increase as rapidly as an ice-cold beer on a hot summer evening. Explain why.
The full solutions are at the end of the unit.
Unit 1: Solutions
Unit 1: Assessment
- c
- d
- .
- q is the heat energy that is transferred because of a difference in temperatures.
- W is energy transferred by a force moving through a distance.
- A closed system is a system where a chemical reaction will take place and the amount of energy in that system will stay the same.
- Internal energy is the kinetic and potential energy of the molecules in the system.
- .
- The tea in the mug is not a closed system. Because there is a wide difference in temperature between the tea and its surroundings, there will be a loss of heat energy from the tea into its surroundings.
- A cold beer in cold conditions will not absorb as much heat energy from its surroundings because there is not such a great difference in the heat energies. Whereas on a hot day, the heat energy difference between the beer and its surroundings is far greater. So, the beer will warm up more quickly because it will absorb more heat energy from the surroundings.
Media Attributions
- Fig 1 © DHET is licensed under a CC BY (Attribution) license
- Fig 2 © pickpik is licensed under a CC0 (Creative Commons Zero) license
- Fig 3 © DHET is licensed under a CC BY (Attribution) license
- Fig 4 © DHET is licensed under a CC BY (Attribution) license
heat force
a chemical reaction that absorbs heat energy from its surroundings
a reaction in which there is an overall release of energy (an increase in temperature of the surroundings)
