Matter and materials: Identify and describe particles
Unit 2: Elements, mixtures, and compounds
Emma Harrage
Unit outcomes
By the end of this unit you will be able to:
- Identify, define, and give examples of:
- a pure substance
- an element
- a compound
- a mixture
- a homogenous mixture
- a heterogenous mixture
- an alloy.
What you should know:
Before you start this unit, make sure you can:
- Identify an atom, ion and a molecule in Subject outcome 5.4, Unit 1: Particles.
Introduction
You will learn to identify, define, and give examples of pure substances that are either elements or compounds, and other substances called mixtures. Mixtures can either be homogenous or heterogenous, in other words, they are either uniform in appearance or not. Alloys are mixtures made up of particular metals and form homogenous mixtures. Brass and steel are examples of alloys.
Elements, compounds, and mixtures
All the objects that we see in the world around us are made of matter. Matter makes up the air we breathe, the ground we walk on, the food we eat and the animals and plants that live around us. Even our bodies are made of matter. Matter can be separated into two different groups, , and .

and are pure substances, which means that they are not mixtures. Pure substances are substances that are made up of only one kind of particle and have a fixed composition.
- Elements are the most basic form of matter. They are made up of one type of atom and cannot be broken down into anything simpler.
- Compounds are made from two or more elements which are chemically joined together. Although compounds can be separated, it is very difficult to do so.
- Mixtures are made up of elements and/or compounds that are not chemically joined together and are easily separated. A mixture can either be or .
Common elements, mixtures and compounds are listed in Table 1.
| Elements | Mixtures | Compounds |
| Gold | Sea water | Water |
| Oxygen | Crude oil | Carbon dioxide |
| Lead | Canned soft drinks | Acetic acid (vinegar) |
| Silver | Air | Sodium chloride (table salt) |
| Mercury | Steel | Iron oxide (rust) |
| Iron | Wine | Hydrochloric acid |
Table 1: Examples of common elements, mixtures, and compounds
Elements
Elements are chemical substances that cannot be divided or changed into other chemical substances by any ordinary chemical means. The smallest unit of an element is the atom. Figure 2 shows a particle diagram of an element.
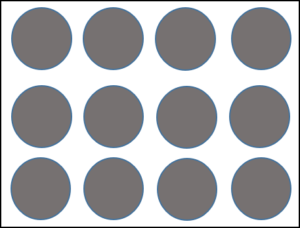
There are 118 known elements. Most of these are natural, but some are man-made.
As we have previously learnt in SO 5.3 Unit 1, elements are found on the periodic table and have a chemical symbol, for example Oxygen (O) and Calcium (Ca).
Compounds
A compound is a chemical substance that forms when two or more different elements combine in a fixed ratio. An important characteristic of a compound is that it has a , which describes the ratio in which the atoms of each element in the compound occur.
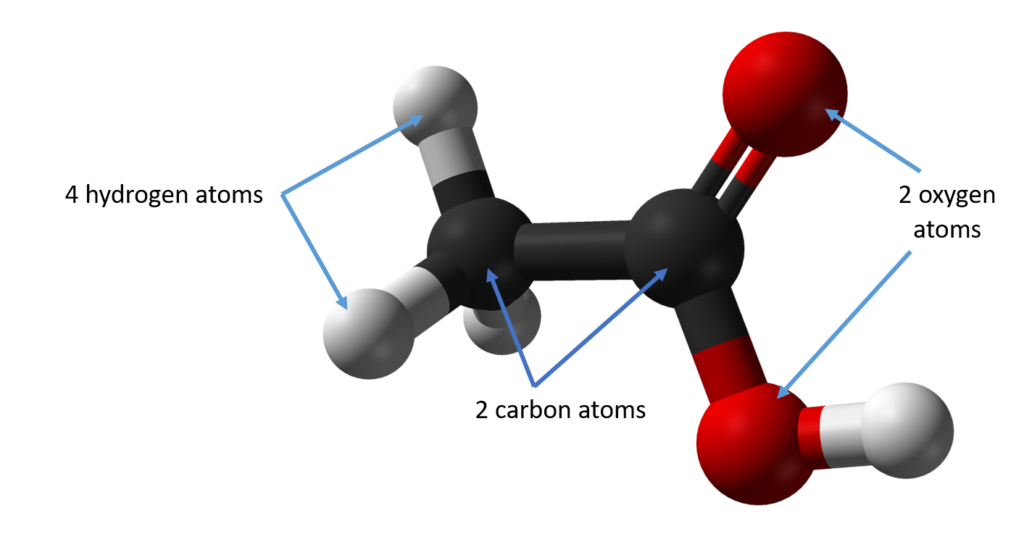
Carbon dioxide has the formula CO2 and is made up of one carbon atom and two oxygen atoms.
Ethanoic acid, which is more commonly known as vinegar, is made up of two carbon atoms, four hydrogen atoms and two oxygen atoms.
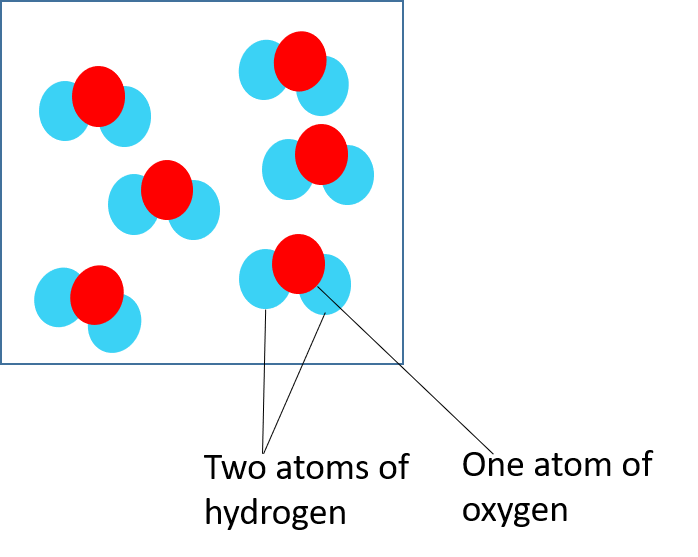
Water is a compound and is made up of two hydrogen atoms for every one oxygen atom.
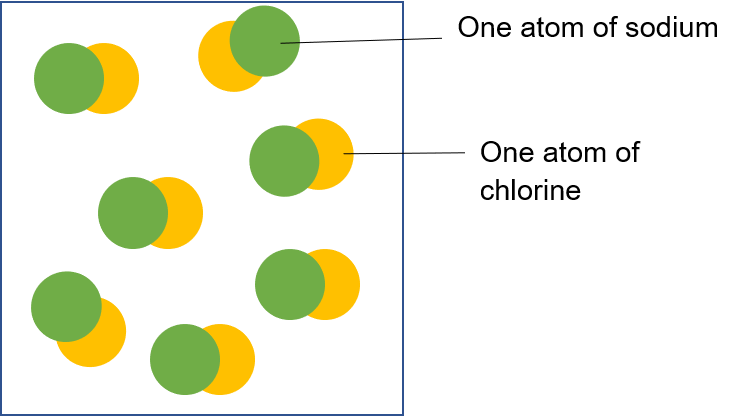
Sodium chloride is a compound made up of one sodium atom for every chlorine atom.
Mixtures
A mixture is a substance which is made up of elements and/or compounds, which are not chemically joined together.
In a mixture, the substances that make up the mixture:
- are not in a fixed ratio – imagine, for example, that you have 250 ml of water and you add sand to the water. It does not matter whether you add 20 g, 40 g, 100 g, or any other mass of sand to the water, it will still be called a mixture of sand and water.
- keep their physical properties – in the example we used of sand and water, neither of these substances has changed in any way when they are mixed together. The sand is still sand and the water is still water.
- can be separated by physical means – to separate something by ‘physical means’ means that there is no chemical process involved. In our sand and water example, it is possible to separate the mixture by simply pouring the water through a filter. Something physical is done to the mixture, rather than something chemical.
Note
If you would like to learn about methods used to separate mixtures, you can watch this video:
Fuse Schools: How to separate mixtures (Duration: 4.07).
Exercise 2.1
State whether the following statements are true of an element, mixture or compound:
- Made up of two or more elements chemically joined together
- Made up of one type of atom and can be found on the periodic table
- Can be separated by mechanical means
- Substance has a chemical formula
- Cannot be broken down into anything simpler
- Made up of elements and/or compounds which are not chemically joined together
The full solutions are at the end of the unit.
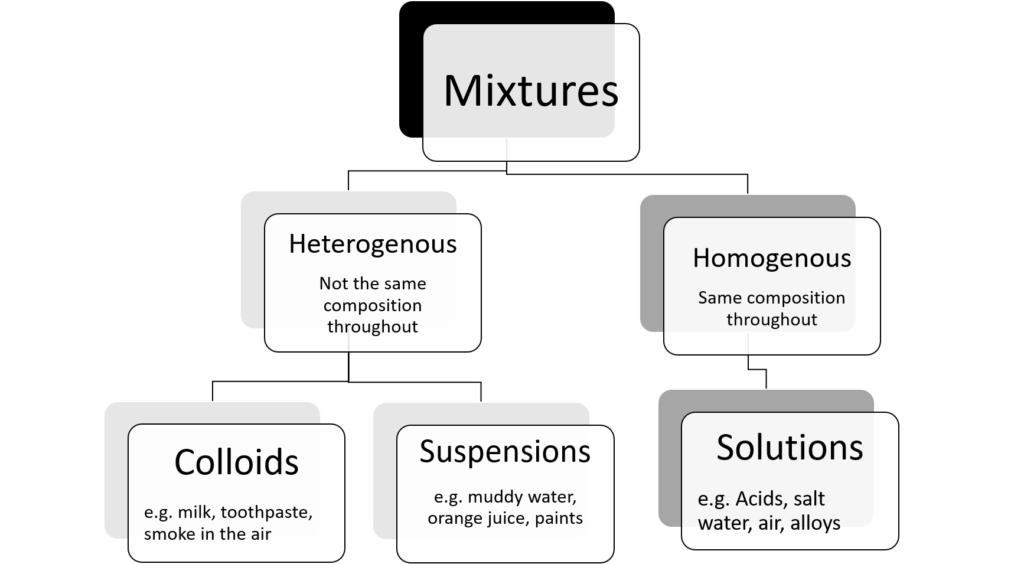
Different types of mixtures
We can group mixtures further by dividing them into those that are heterogeneous and those that are homogeneous.
Homogenous mixtures
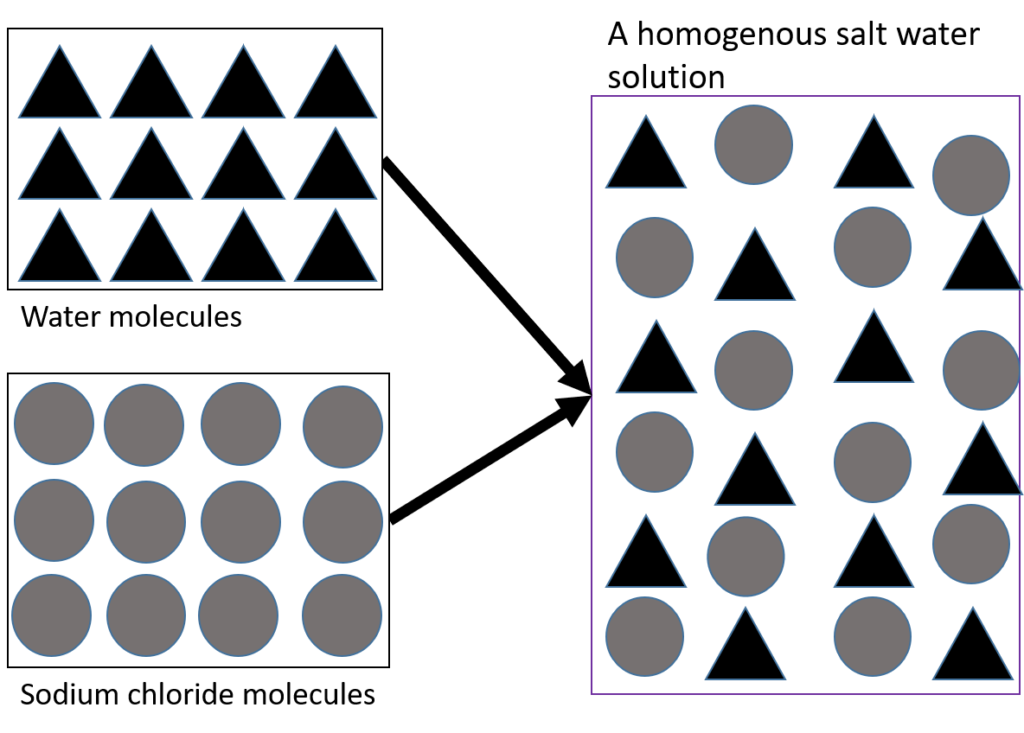
A homogeneous mixture has a definite composition and specific properties. In a homogeneous mixture, the different parts cannot be seen. Homogenous mixtures are mixtures where all the substances which make up the mixture are in the same state. A homogenous mixture of a solid and a suitable will form a solution, as will a mixture of gases like the air.
Salt water is a solution of salt dissolved in water and is an example of a homogeneous mixture. Figure 7 shows that when the salt (sodium chloride) dissolves, its particles spread evenly through the water so that all parts of the solution are the same, and you can no longer see the salt as being separate from the water.
The air we breathe is another example of a homogeneous mixture as it is made up of different gases which are in a constant ratio, and which cannot be visually distinguished from each other. In other words, you cannot see the different components. Homogenous mixtures tend to be transparent, and can be either solids, liquids or gases.

are homogenous mixtures of different metals, and in some instances non-metals, used to make a variety of objects including coins, car wheels and bells.
| Common alloys | Elements used | Uses |
| Bronze | Copper and tin | Statues |
| Brass | Copper and zinc | Bells |
| Steel | Iron, carbon, chromium, nickel, silicon, manganese, boron and others | Structures, buildings, machinery, cutlery |
| Solder | Tin, lead and antimony | Welding |
| Alnico | Aluminium, nickel and cobalt | Magnets |
Heterogeneous mixtures
A heterogeneous mixture does not have a definite composition. Cereal in milk is an example of a heterogeneous mixture. Soil is another example. Soil has pebbles, plant matter and sand in it. Although you may add one substance to the other, they will stay separate in the mixture. We say that these heterogeneous mixtures are non-uniform, in other words they are not exactly the same throughout.

 Figure 9: Milk is a heterogenous mixture, and so is oil and water
Figure 9: Milk is a heterogenous mixture, and so is oil and water
Milk is a heterogenous mixture, and so is oil and water. Milk is a of water proteins, fats, lactose, and minerals. The proteins, fats, lactose, and minerals are spread out though the water. The oil and water mixture is also a colloid because the oil and water do not mix and the oil ‘sits’ on top of the water. A common example of a suspension is muddy water. A is a mixture of solid particles in a liquid which will settle out quickly once the liquid is still.

Note
Useful things to remember:
- A heterogeneous mixture is a mixture in which the composition is not uniform throughout the mixture.
- Heterogenous mixtures can be made up of substances in different phases.
- A homogeneous mixture is a mixture in which the composition is uniform throughout the mixture.
- Homogenous mixtures are all in the same phase.
- All solutions would be considered homogeneous.
Note
If you need further explanation on homogenous and heterogenous mixtures, you can watch this video:
Robin Reaction: Homogenous and heterogenous mixtures! (Duration: 7.38).
Summary
In this unit you have learnt the following:
- All the objects and substances that we see in the world are made of matter.
- This matter can be classified according to whether it is a mixture or a pure substance.
- A mixture is a combination of two or more substances, where these substances are not bonded (or joined) to each other and no chemical reaction occurs between the substances. Examples of mixtures are air (a mixture of different gases) and cereal in milk.
- The main characteristics of mixtures are that the substances that make them up are not in a fixed ratio, these substances keep their physical properties, and these substances can be separated from each other using mechanical means.
- A heterogeneous mixture is one that consists of two or more substances. It is nonuniform and the different components of the mixture can be seen. An example would be a mixture of sand and water.
- A homogeneous mixture is one that is uniform, and where the different components of the mixture cannot be seen. An example would be salt in water.
- Pure substances can be further divided into elements and compounds.
- An element is a substance that cannot be broken down into other substances through chemical means.
- All the elements are found on the periodic table. Each element has its own chemical symbol. Examples are iron (Fe), sulfur (S), calcium (Ca), magnesium (Mg) and fluorine (F).
- A compound is a substance made up of two or more different elements that are joined together in a fixed ratio. Examples of compounds are sodium chloride (NaCl), iron sulfide (FeS), calcium carbonate (CaCO3) and water (H2O).
Unit 2: Assessment
Suggested time to complete: 25 minutes
- Copy and complete the following table into your notebook.
Substance Element, mixture, or compound? Type of mixture (if applicable) Water Brass Jelly Aluminium Table salt Sugar water Cooking oil - In your notebook, draw particle diagrams to show an element, a heterogenous mixture and the compound water.
- Give a one-word term for the following:
- a mixture with a uniform composition
- a substance made up of only one type of atom
- a substance which has a chemical formula
- a mixture where undissolved substances are spread throughout the substance
The full solutions are at the end of the unit.
Unit 2: Solutions
Exercise 2.1
- compound
- element
- mixture
- compound
- element
- mixture
- .
Substance Element, mixture, or compound? Type of mixture (if applicable) Water Compound N/A Brass Mixture homogenous Jelly Mixture heterogenous Aluminium Element N/A Table salt Compound N/A Sugar water Mixture homogenous Cooking oil Mixture homogenous - .
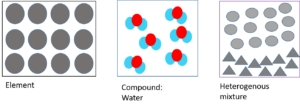
- .
- homogenous
- element
- compound
- colloid/heterogenous
Media Attributions
- Matter flow diagram © Siyavula is licensed under a CC BY (Attribution) license
- Diagram of an element © Department of Higher Education and Training is licensed under a CC BY (Attribution) license
- acetic acid © Department of Higher Education and Training is licensed under a CC BY (Attribution) license
- Diagram of water © Department of Higher Education and Training is licensed under a CC BY (Attribution) license
- Diagram of sodium chloride © Department of Higher Education and Training is licensed under a CC BY (Attribution) license
- mixtures flow diagram © Department of Higher Education and Training is licensed under a CC BY (Attribution) license
- A homogenous mixture © Department of Higher Education and Training is licensed under a CC BY (Attribution) license
- Coloured solutions © Benjah-bmm27 is licensed under a CC0 (Creative Commons Zero) license
- milk-bottle © Wolfgang Eckert is licensed under a CC0 (Creative Commons Zero) license
- oil and water © andrea candraja is licensed under a CC0 (Creative Commons Zero) license
- Muddy water © mohann-8137807 is licensed under a CC BY-SA (Attribution ShareAlike) license
- Element, compound mixture particle diagrams © Department of Higher Education and Training is licensed under a CC BY (Attribution) license
substances with two or more elements and/or compounds which are not chemically joined together and can be easily separated
substances that are made up of only one kind of particle and have a fixed composition
substances that cannot be broken down into other substances through chemical means
substances made up of two or more different elements that are joined together in a fixed ratio
uniform in composition
not uniform in composition
notation that shows the number of atoms and the names of atoms in a compound
dissolves in a suitable solvent
a liquid or gas into which a soluble substance can dissolve
metallic substances composed of two or more elements
a mixture which has suspended particles; to qualify as a colloid the mixture must be one that does not settle or would take a very long time to settle appreciably
a mixture in which the solute particles do not dissolve, but get suspended throughout the bulk of the solvent and will settle out quite quickly.
