Matter and materials: Identify and describe particles
Unit 1: Particles
Emma Harrage
Unit outcomes
By the end of this unit you will be able to:
- Identify and define:
- an atom
- an ion
- a molecule.
What you should know:
Before you start this unit, make sure you can:
- Confidently complete all the work in Subject outcome 5.2.
- Confidently complete all the work in Subject outcome 5.3.
Introduction
In this unit you will learn to identify and define an atom, an ion, and a molecule. An atom is the simplest form of matter and an ion forms when an atom loses or gains an electron. A molecule is made up of two or more atoms joined together and can either become a compound or remain an element.
Atoms
In Unit 1 of SO 5.2, we learnt about atoms. An is the smallest form of matter and is made up of electrons, protons and neutrons. The negatively charged electrons orbit the nucleus of the atom. Protons are positively charged particles and neutrons are neutral particles, and they are found in the nucleus of an atom.
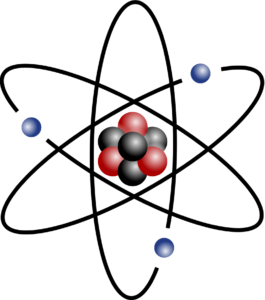
Each atom of an element has the same number of protons and electrons. The number of protons is the atomic number. For a neutral atom, the number of electrons is the same as the number of protons, as the charge on the atom must balance. For example, each atom of the element carbon has 6 protons, 6 electrons and 6 neutrons. Lithium has 3 protons, 3 electrons and 4 neutrons.
Ions
During chemical bonding, atoms will lose or gain valence electrons. This does not change the type of atom it is, but it changes the charge of the atom. If electrons are added, then the atom will become more negative. If electrons are taken away, then the atom will become more positive. The atom that is formed in either of these two cases is called an . An ion is a charged atom.
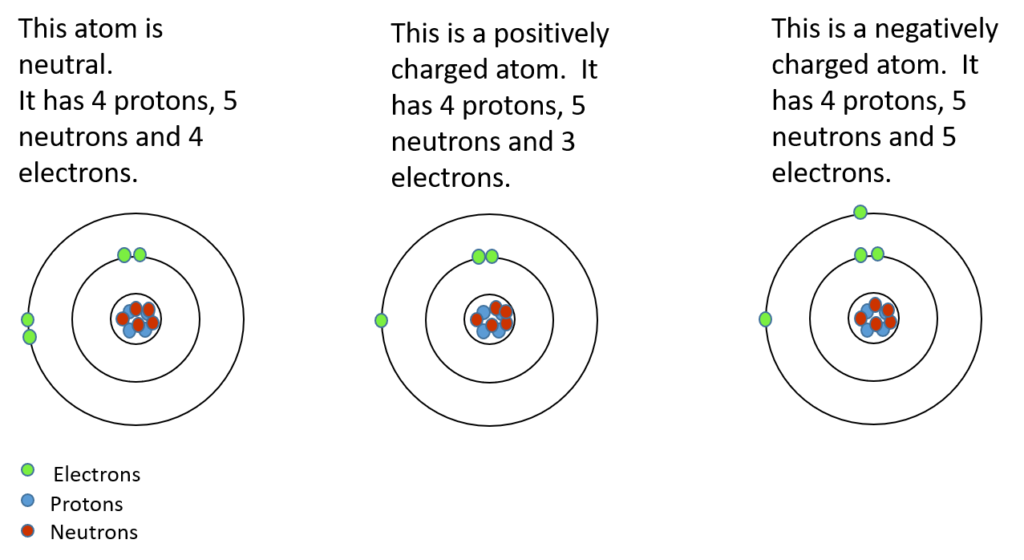
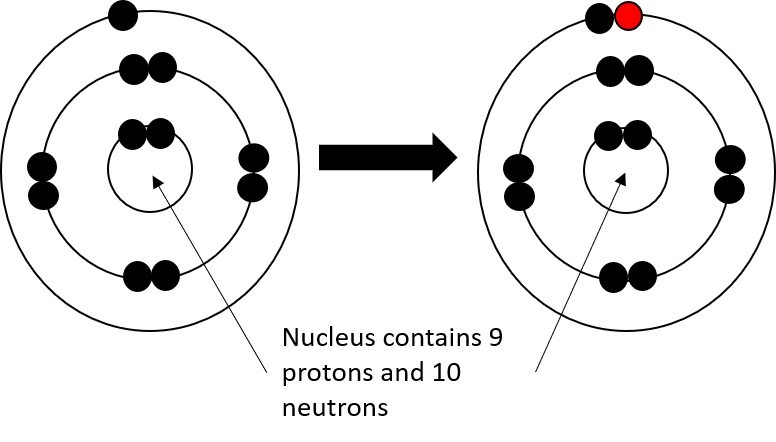
Activity 1.1: Build an ion
Time required: 15 minutes
What you need:
- an internet connection
What to do:
You will build ions online using a program.
- Go to this Build an Atom simulation and click on the ‘Build an atom’ game.
- Click on Atom on the home screen.
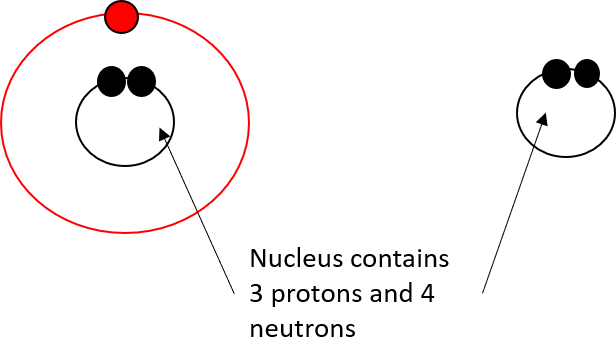
- Add 3 electrons, 3 protons, and 4 neutrons to the shells to create a lithium atom.
- Remove an electron to create a lithium ion with a positive charge, Li+.
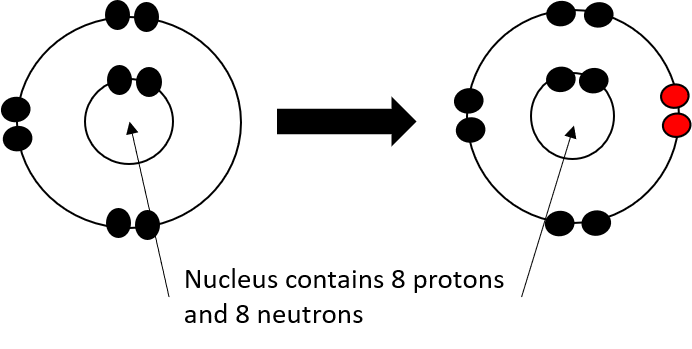
- Add 8 neutrons, 8 protons and 8 electrons to the shells to create an oxygen atom.
- Remove 2 electrons to create an O2- ion.
- Repeat this process for the following elements:
- Na
- F
- Mg
- B
Exercise 1.1
- Which of the following elements are ions?
- Be
- Mg2+
- O2-
- Write a definition for an ion.
- Describe the difference between an atom and an ion.
The full solutions are at the end of the unit.
Molecules
A can be defined as two or more atoms bonded together. A molecule does not have a charge. A molecule can be made up of the same element or different elements.
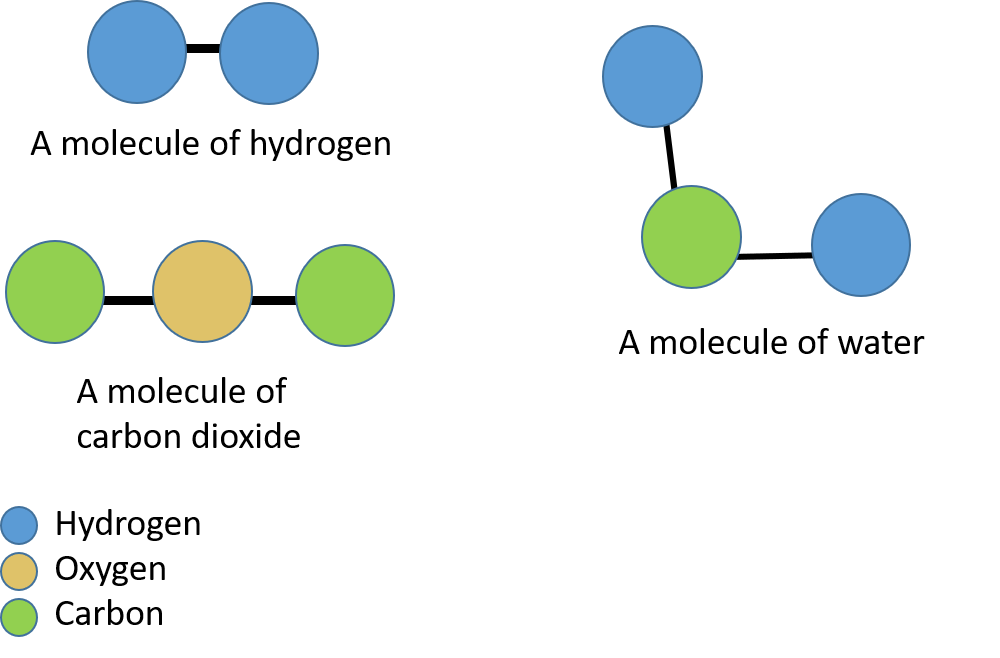
In Figure 6 you can see molecules of hydrogen, water and carbon dioxide. The hydrogen molecule is made up of two hydrogen atoms. The carbon dioxide molecule is made up of one carbon atom and two oxygen atoms. The water molecule is made up of one oxygen atom and two hydrogen atoms.
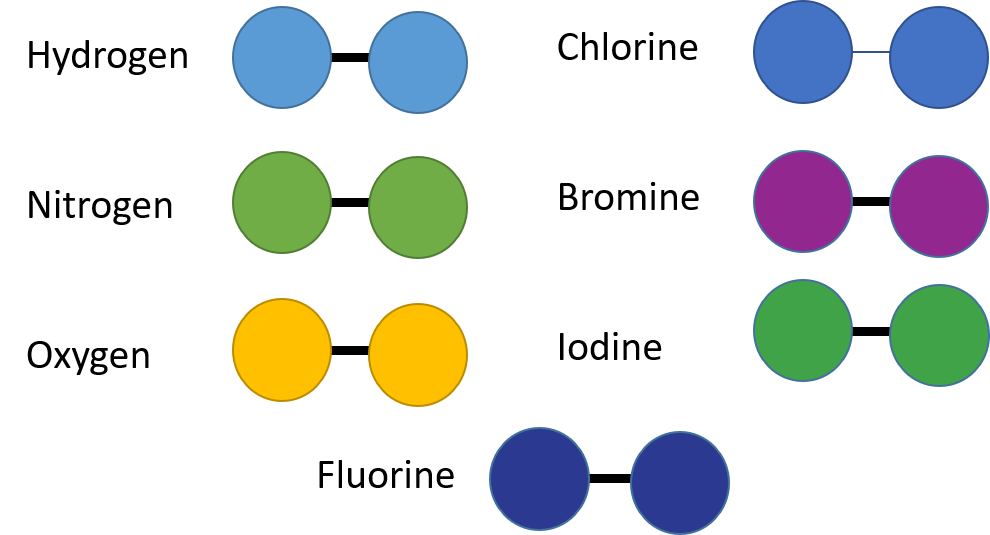
There are seven elements which naturally form molecules and are called . They are all non-metals and most of them are halogens. These are shown in Figure 7.
Activity 1.2: Building molecules
Time required: 15 Minutes
What you need:
- an internet connection
What to do:
- Go to this Build a Molecule simulation and click on the ‘Build a molecule’ game.
- Open the ‘Make Molecules’ tab.
- With kit 1, build a hydrogen molecule using the atoms provided and drag it into the hydrogen window on the right.
- Select kit 2 by pressing the yellow arrow on the bottom and make a molecule of water and drag it into the correct window.
- Select kit 3 and make molecules of carbon dioxide and nitrogen.
- Click on ‘collect multiple’ on the top row and complete the molecules.
Summary
In this unit you have learnt the following:
- Atoms are the most basic form of matter and are made up of electrons, protons and neutrons.
- If an atom loses or gains electrons to gain a stable electron configuration, it is called an ion.
- If an atom gains electrons it will have an overall negative charge.
- If an atom loses electrons it will have an overall positive charge.
- A molecule is made up of two or more atoms joined together. These atoms can be atoms of the same element or atoms of different elements. If they are made of the same elements, they are called diatomic elements.
Unit 1: Assessment
Suggested time to complete: 10 minutes
- Ions are:
- atoms with a positive or negative charge
- atoms with no charge
- atoms with ONLY a positive charge
- atoms with ONLY a negative charge
- If an element has 3 valence electrons, what charge will likely form on its ion? Hint: It will lose those electrons. What happens to the charge when it loses 3 electrons?
- +3
- +5
- -3
- -5
- Molecules are made up of more than one _______.
- electron
- proton
- atom
- element
- What is the name given to a molecule made up of two atoms of the same element?
The full solutions are at the end of the unit.
Unit 2: Solutions
Exercise 1.1
- b and c – because they have a positive charge or a negative charge.
- An ion is an atom which has either gained or lost electrons. It will have a positive charge if it has lost electrons or a negative change if it has gained electrons.
- An atom has a neutral charge because it has the same number of electrons and protons. An ion has the same number of protons and neutrons as when it was a neutral atom, but has either gained or lost electrons giving it a charge.
Unit 2: Assessment
- a – ions are atoms with a positive or negative charge
- a – the charge becomes +3 when it loses 3 electrons.
- c – atom
- diatomic elements
Media Attributions
- An atom © indolences is licensed under a CC BY-SA (Attribution ShareAlike) license
- ions © Department of Higher Education and Training is licensed under a CC BY (Attribution) license
- Lithium losing electron © Department of Higher Education and Training is licensed under a CC BY (Attribution) license
- oxygen ion © Department of Higher Education and Training is licensed under a CC BY (Attribution) license
- fluorine ion © Department of Higher Education and Training is licensed under a CC BY (Attribution) license
- Molecules © Department of Higher Education and Training is licensed under a CC BY (Attribution) license
- Diatomic elements © Department of Higher Education and Training is licensed under a CC BY (Attribution) license
simplest form of matter; made of equal numbers of protons, electrons, and neutrons
a charged atom due to the loss or gain of valence electrons
two or more atoms bonded together
two atoms of the same element which naturally form a molecule
