Chemical change: Differentiate between physical and chemical change
Unit 2: Separating mixtures
Emma Harrage
Unit outcomes
By the end of this unit you will be able to:
- Separate a mixture by using either chemical or physical methods, such as:
- manual methods
- filtration
- magnetism
- chromatography
- fractional distillation
- precipitation reactions
- separating funnel.
What you should know:
Before you start this unit, make sure you can:
- Confidently complete all the work in Subject outcome 5.4 Unit 1: Particles.
- Confidently complete all the work in Subject outcome 5.4 Unit 2: Elements, mixtures and compounds.
Introduction
In this unit you will learn about both the physical and chemical separation of mixtures. Physical separation does not change the chemical composition of the substances being separated. Physical separation can involve a change of state. Chemical separation occurs as a result of a chemical reaction.
Separating mixtures
A mixture is a made up of different elements and/or compounds which are not chemically joined and so they can be separated into different substances. Mixtures can be sorted physically or chemically.
Physical separation techniques
There are a few different ways that mixtures can be separated using physical methods.
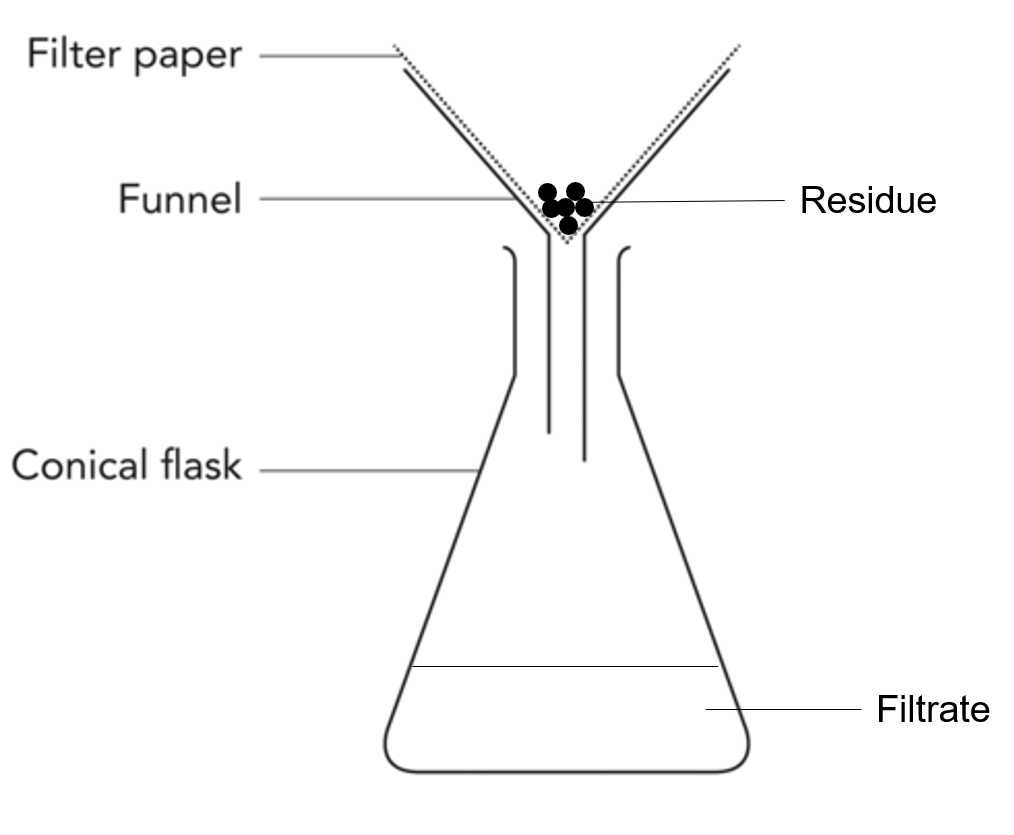
Filtration
Filtration involves separating an insoluble solid from a mixture, for example sand and water, mud and water, tea leaves from water.
Swimming pool water is filtered through a sand filter to remove insoluble debris and air can be filtered through membranes to remove dust and other particles.
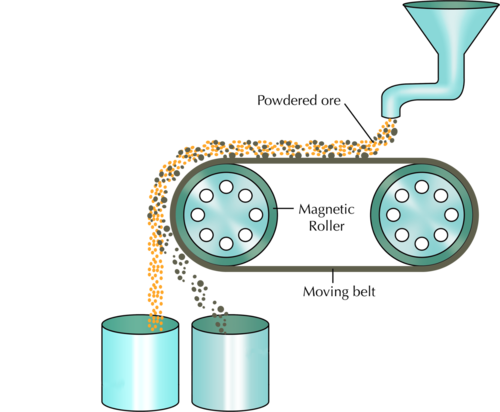
Magnetism
Magnetism involves separating a magnetic substance from a non-magnetic one using a magnet.
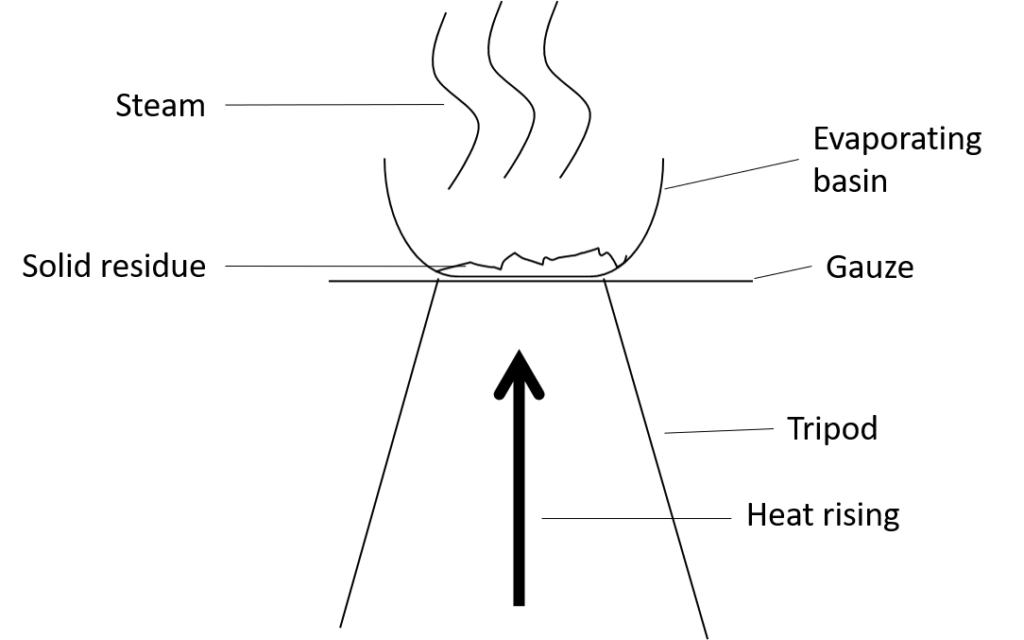
Evaporation
Evaporation involves separating a soluble solid from a liquid, for example salt from sea water.
Evaporation works by allowing the liquid to change into a gas leaving the solid that was dissolved behind. Using heat will speed up the process.

Simple distillation
Simple distillation is the separation of a mixture of liquids with different boiling points. The liquid with the lowest boiling point will boil off first, leaving behind the liquid with the higher boiling point.
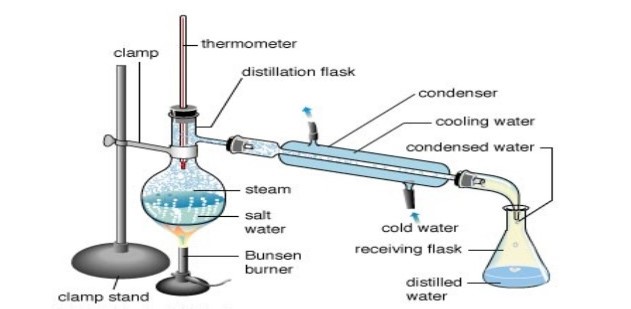
The simple distillation process:

Alcoholic drinks such as whiskey and brandy are distilled to separate the alcohol from water. Alcohol has a lower boiling point than water. Many distilleries use huge copper vats called stills to boil off the alcohol from the mixture.

Fractional distillation
Fractional distillation separates a mixture into several different parts, called fractions.
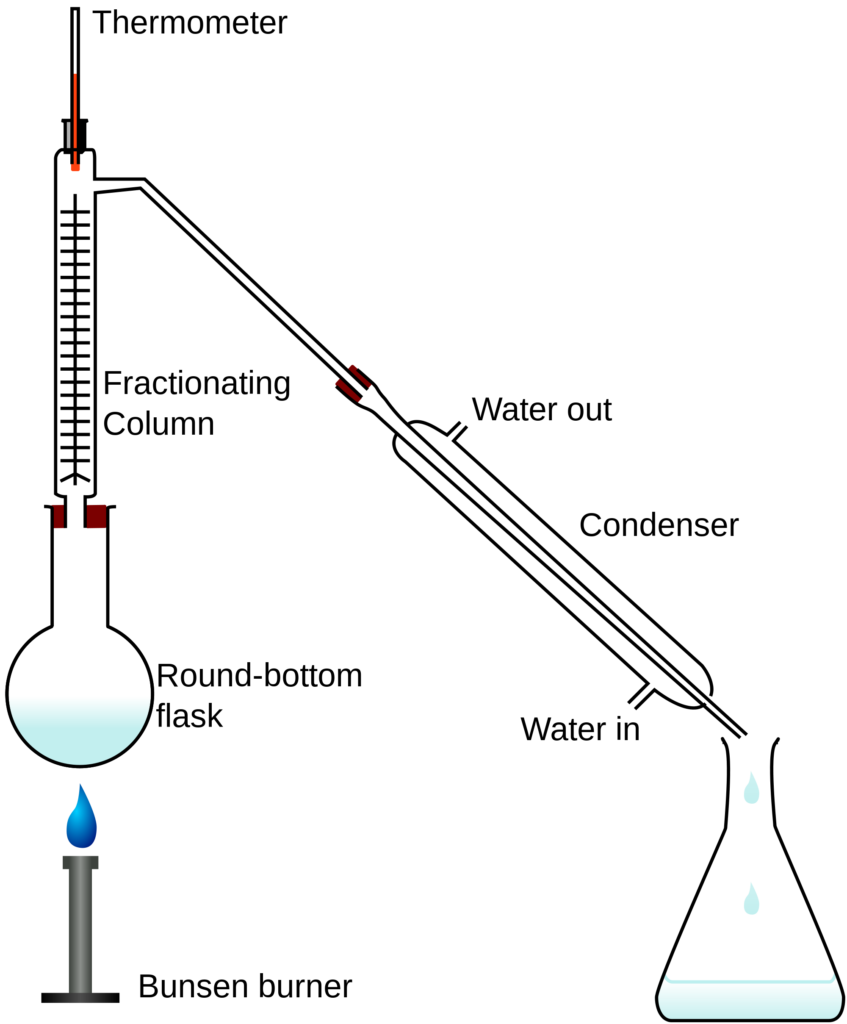
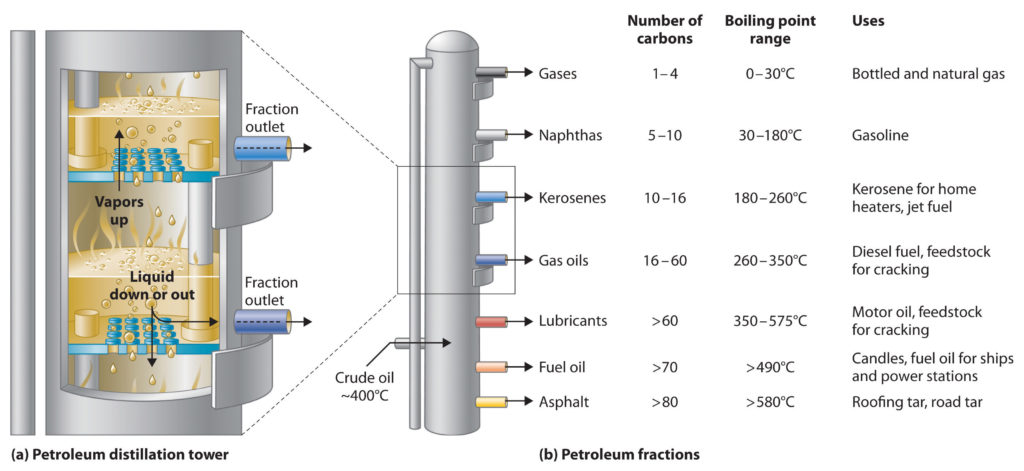
Crude oil is distilled using fractional distillation. Crude oil is a mixture consisting of chains of carbon atoms of different lengths. The length of the carbon chain determines its boiling point. The crude oil is evaporated, and its vapours condense at different temperatures in the fractionating column. Each fraction contains hydrocarbon molecules with a similar number of carbon atoms and a similar range of boiling points.
A tall fractionating column is fitted above the heated mixture, with several condensers coming off at different heights. The column is hot at the bottom and cool at the top. Substances with high boiling points, those with longer carbon chains, condense at the bottom and substances with lower boiling points, those with shorter carbon chains, continue to rise and condense at various other points along the condenser.
Note
For further explanation about fractional distillation, watch this video:
Fuse Schools: Fractional Distillation (Duration:4.05).
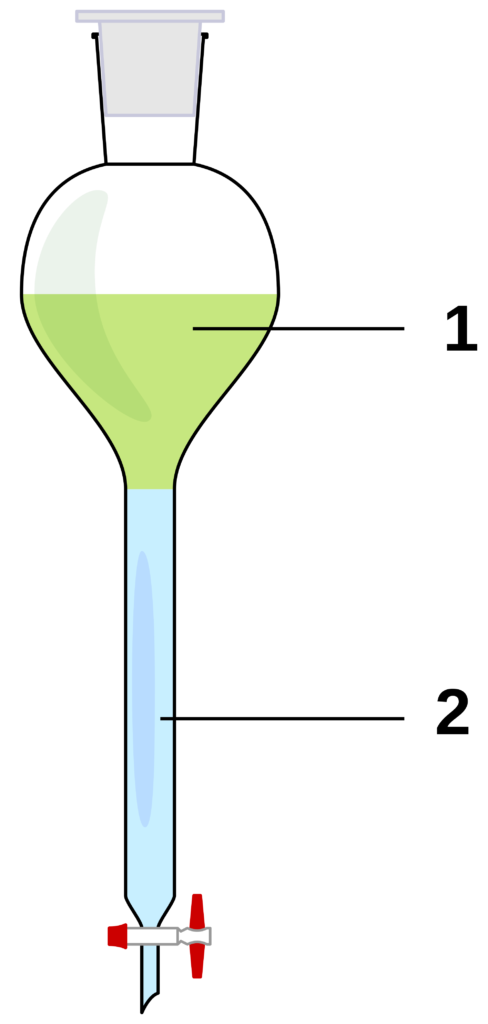
Separating funnel
A separating funnel can be used to separate two liquids.
A separating funnel is a glass funnel with a tap at the bottom. The mixture of liquids is placed inside the separating funnel. The liquid with the lower density floats on top. When the tap is opened, the liquid with the higher density starts to flow through the separating funnel into the container. The tap is closed when the transition point between the two layers is just above the tap. Using another container, the rest of the top layer and the first part of the second layer is allowed to run through the tap. This sample is discarded. The rest of the second liquid is then allowed to run into a third container.
In Figure 10 you can see that 1 shows the less dense liquid and 2 shows the denser liquid that can be removed first.
Exercise 1.1
- In a coffee machine, the ground coffee is separated from the coffee solution by using:
- toilet paper
- tissue paper
- filter paper
- sandpaper
- Evaporation is used to:
- separate solids of different particle size.
- obtain the solute from the solution.
- separate liquids of different boiling points.
- separate the dyes in a marker.
- Water and alcohol are easily separated by distillation because of their:
- different colours
- different boiling points
- different melting points
- different densities.
- Which one of the following pairs of separation techniques will BOTH separate salt from a mixture of salt and water?
- decanting and filtration
- distillation and evaporation
- decanting and distillation
- chromatography and evaporation.
- Which one of the following is a disadvantage of evaporation?
- the solvent is not recovered
- it always requires heat
- it cannot be used for insoluble solids
- all the solute is recovered
The full solutions are at the end of the unit.
Chemical separation techniques
Chemical separation is when you use a chemical substance to separate a mixture.
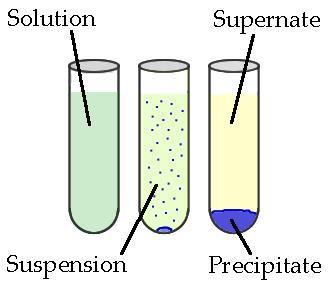
A primary way that mixtures can be separated using chemical separation. This is called precipitation. A precipitation reaction is the formation of an insoluble salt when two solutions containing soluble salts are combined. The insoluble salt that settles in a solution is known as the . The solids produced in precipitate reactions are crystalline solids and can be suspended throughout the liquid or settle at the bottom of the solution. The remaining fluid is called supernatant liquid. The two components of the mixture (precipitate and ) can be separated by various methods, including filtration.
The following reaction between silver nitrate and copper is a common precipitation reaction:
[latex]\scriptsize \text{Cu+2AgN}{{\text{O}}_{\text{3}}}\to \text{2Ag+Cu(N}{{\text{O}}_{\text{3}}}{{\text{)}}_{\text{2}}}[/latex]
A piece of copper suspended in aqueous silver nitrate will cause the silver to precipitate out of the solution and form on the wire. The solution will go blue because the copper reacts with the nitrate ions (NO3)– to form copper nitrate. Copper is more reactive than silver which is why it will the silver from the solution.

Activity 1: Precipitation of copper
Time required: 15 minutes
What you need:
- 25 g copper sulfate
- 2 iron nails
- hot water
- a glass or transparent beaker
- string
- a pencil or stick
What to do:
- Dissolve 25g of copper sulfate into 100 ml of hot water in the glass.
- Stir the mixture until it has dissolved.
- Tie the two iron nails together with the string and suspend them from the pencil or stick lying on top of the glass. The nails must be suspended in the copper sulfate solution.
- Leave the reaction for 24 hours and note your observations.
What did you find?
Copper is a reddish-brown colour, and it will precipitate out of the solution and begin to form on the nails. The solution will also change colour from blue to a greenish colour as the iron dissolves into the solution to form iron sulfate. This precipitation reaction happens because copper is less reactive than iron.
Chromatography
Chromatography is used to separate a mixture according to the solubility of the dissolved molecules. Paper chromatography has two phases, the stationary phase which is the paper and the mobile phase which is the solvent being used. The mixture is placed on the paper a little way from the edge and then the lower edge of the paper is placed in the solvent. The components of the mixture will separate on the paper and show up as different spots. This is called a chromatogram. An impure substance will therefore produce a chromatogram with more than one spot. If two or more mixtures have the same components, they will produce identical chromatograms.
The solvents used in chromatography are water, if the substance you are separating is water soluble, and if the substance is insoluble then ethanol is used.
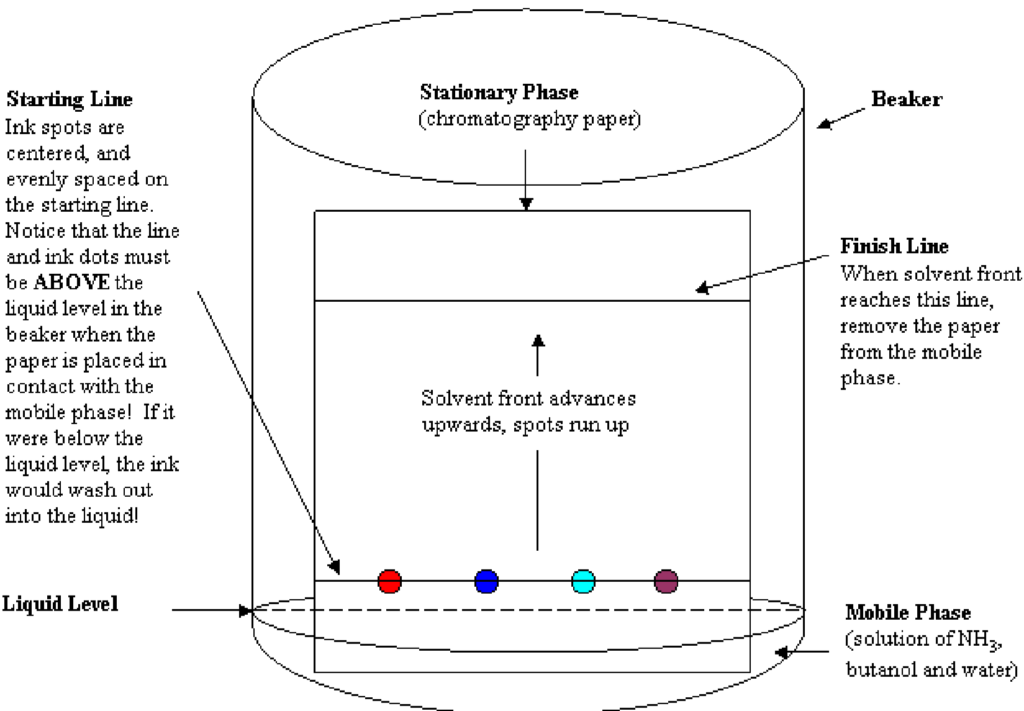
The unknown components of mixtures can be identified by comparing them with chromatograms of known substances.
Paper chromatography is carried out on the unknown sample and a sample of a known compound. This is done simultaneously on the same paper using the same solvent. If the resulting spot(s) are at the same height, then the substance being tested is the same as the known substance.
Activity 1: Separating inks in kokis
Time required: 30 minutes
What you need:
- different coloured kokis or food colouring (hint: Black, brown, green, dark blue and purple are the most successful colours to use)
- white coffee filters
- a medicine dropper
- transparent glass
- water
- scissors
- a pencil
- sticky tape
What to do:
Part 1:
- Take 1 coffee filter, in the centre draw a blob of ink using a koki or a drop of food colouring.
- Place it over the glass.
- Using the medicine dropper, carefully drop 1 drop of water on the centre of the ink blob.
- Add a further drop of water once the ink has stopped moving and continue until the ink does not move anymore. Be careful to only add the water to the centre of the ink blob.
Part 2:
- Fill the glass with approximately 1 centimetre of water.
- Cut a long thin strip of filter paper. Draw a pencil line approximately 1 centimetre from the bottom of the paper.
- Draw a blob of ink on the pencil line using a koki or a drop of food colouring.
- Secure the top of the piece of paper around the pencil with tape and hang the paper in the glass making sure the paper reaches the water but the water must not touch the ink.
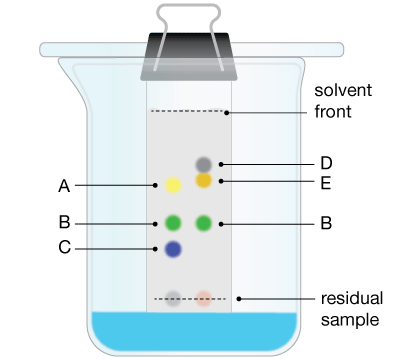
What did you find?
As the ink dissolves in the water soaking the filter paper, it begins to spread out along the filter paper. This is because the ink is soluble in the water and the ink will separate out into the different coloured inks it is made of according to the solubility of the particles. The further the particles travel the more soluble they are in the solvent.
Summary
In this unit you have learnt the following:
- Chemical reactions involve large changes in energy. Chemical reactions are not easily reversible.
- Mixtures can be separated physically or chemically.
- Chemical methods of separation include precipitation reactions.
- Physical methods of separation include:
- chromatography
- simple and fractional distillation
- evaporation
- filtration
- magnetic separation.
Unit 2: Assessment
Suggested time to complete: 20 minutes
- Think carefully about the following statements. Are they true or false?
- In filtration, the filtrate is always a pure liquid.
- Drinking water can only be obtained from seawater by distillation.
- The fractional distillation of liquids is only possible if the liquids have different boiling points.
- Paper chromatography is a physical method of separating mixtures.
- Mixtures have fixed melting and boiling points.
- Name the technique most suited to separate the following mixtures:
- To obtain drinking water from muddy water.
- To separate petrol from crude oil.
- To obtain pure sugar from a solution.
- To determine whether the colouring in a fruit juice is a single substance or a mixture of coloured substances.
- Distillation can be used to separate a mixture of liquids with different boiling points, or a solid from a liquid where the solvent is recovered.
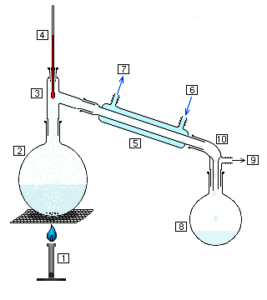
- Name the separation process shown in the diagram.
- Explain the benefit of using distillation to separate a soluble solid from a liquid, as opposed to evaporation.
- Name the item labelled 5 in the diagram.
- Identify the part 6 or 7 of item 5 which is connected to the cold tap.
- How could you show that the water collected contains no salt?
- Paper chromatography can be used to separate the dyes in a sample of ink.
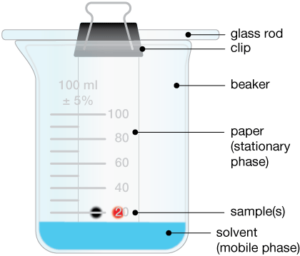
- The ink spot is placed on the chromatography paper just above the level of the solvent. Why?
- What happens to the ink spot as the water moves up the paper?
- What would happen to a spot of water-soluble ink consisting of a single-coloured dye if it were used in the above experiment?
- Is a precipitation reaction a chemical or physical change? Explain your answer.
The full solutions are at the end of the unit.
Unit 2: Solutions
Exercise 2.1
- c
- b
- b
- b
- a
Unit 2: Assessment
- .
- false
- true
- true
- true
- false
- .
- distillation/filtration
- fractional distillation
- evaporation
- chromatography
- .
- simple distillation
- the solvent is recovered
- condenser
- water in
- boil the water; if the water is pure then it will boil at 100 0C at sea level
- .
- so the ink does not flood into the water
- the ink dissolves into the solvent and is carried up the paper
- it would only make 1 spot on the paper
- Displacement reactions are chemical changes because a new substance is made.
Media Attributions
- Filtration apparatus © Department of Higher Education and Training is licensed under a CC BY (Attribution) license
- Magnetic roller © Siyavula is licensed under a CC BY (Attribution) license
- Evaporation apparatus © Department of Higher Education and Training is licensed under a CC BY (Attribution) license
- Salt pans © World Imaging is licensed under a CC BY-SA (Attribution ShareAlike) license
- Simple distillation © H Padleckas is licensed under a CC0 (Creative Commons Zero) license
- Distillation flow diagram © Department of Higher Education and Training is licensed under a CC BY (Attribution) license
- distillery © Bernd Hildebrandt is licensed under a CC0 (Creative Commons Zero) license
- Fractional distillation apparatus © Theresa knott is licensed under a CC BY-SA (Attribution ShareAlike) license
- crude oil © Libretext is licensed under a CC BY-NC (Attribution NonCommercial) license
- separating funnel © Shakki is licensed under a CC BY-SA (Attribution ShareAlike) license
- precipitation_reactions © MarcoSwart is licensed under a CC0 (Creative Commons Zero) license
- Copper and silver nitrate © Libretext is licensed under a CC BY-NC (Attribution NonCommercial) license
- paper chromatography © Libretext is licensed under a CC BY-NC (Attribution NonCommercial) license
- Chromatography © Dr Jeff Ctuzan is licensed under a CC BY-NC (Attribution NonCommercial) license
- numbered distillation apparatus © H PadleckasCC by NC is licensed under a CC BY-NC (Attribution NonCommercial) license
- PaperChromSetup © Dr Jeff Ctuzan is licensed under a CC BY-NC (Attribution NonCommercial) license
will not mix.
the solid that forms in a solution during a chemical reaction
liquid found above a precipitate
take the place of something else.
