Chemical change: Identify, describe, and apply principles of heat
Unit 2: Heat and heat transfer
Emma Harrage
Unit outcomes
By the end of this unit you will be able to:
- Understand what heat is.
- Understand the three scales of measurement used to measure heat.
- Understand that heat can be transferred by:
- convection
- conduction
- radiation.
What you should know
Before you start this unit, make sure you can:
- Summarise the principles of the first law of thermodynamics. To revise this, go to Subject outcome 6.1, Unit 1.
- Explain waves. To revise this, go to Subject outcome 3.1, Unit 1.
- List the wave properties of light. To revise this, go to Subject outcome 3.2, Unit 1.
Introduction
In this unit you will learn what heat is, the different scales used to measure heat and how heat is transferred. Heat is a type of energy that can be transferred in three ways: by convection currents where air rises as it is heated and drops as it cools, by radiation which is direct heating and by conduction which is where heat is spread throughout an object by the movement of particles.
Heat
When you think of , you think of a very hot day in the middle of summer. But heat is a type of energy. Heat is energy transfer to or from a thermodynamic system.
In thermodynamics, energy transferred as heat contributes to change in a system’s .
The quantity of energy transferred as heat can be measured by its effect on the states of interacting bodies. For example, heat transfer can be measured by the amount of ice melted, or by change in of a body or in the surroundings of the system.
Heat is caused by the flow of from one body to another because there is a difference in temperature. Heat will usually flow from an object with a higher temperature to an object with a lower temperature. Thermal energy always flows from warmer areas to cooler areas. Heat is transferred through , , or .
The conventional symbol used to represent the amount of heat transferred in a thermodynamic process is Q. The SI unit of heat is the joule (J).
Note
Temperature is the average kinetic energy of the particles within a given object and is measured by three scales of measurement (Fahrenheit, Celsius, Kelvin).
Thermal energy is defined as the total kinetic energy within a given system.
It is important to remember that heat is caused by the flow of thermal energy due to differences in temperature.
Heat and temperature
Temperature is a physical quantity that expresses hot and cold. Thermal energy, present in all matter, is the source of heat, and it flows between bodies when one body comes in contact with another body that is either colder or hotter.
To measure the amount of heat a system has, we can use a thermometer. A thermometer measures the temperature of a system. Thermometers are calibrated in various temperature scales. The most common scales are the Celsius scale (formerly called centigrade, denoted as °C), the Fahrenheit scale (denoted as °F), and the Kelvin scale (denoted as K). The Kelvin scale is predominantly used for scientific purposes according to the International System of Units (SI).
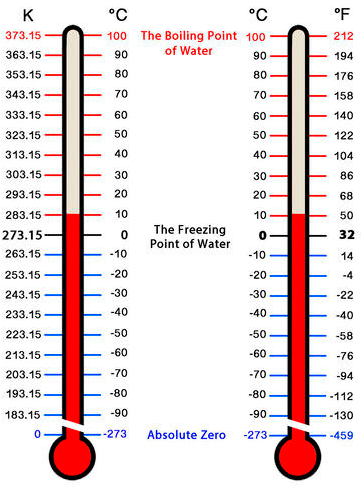
Most countries use the Celsius scale. The Celsius scale (symbolised by °C and called “degrees Celsius”) defines 0 °C as the freezing point of water and 100 °C as the boiling point of water at sea level. This scale is divided into 100 divisions between these two points but can extend higher and lower.
Other countries, like the United States of America, use the Fahrenheit scale (symbolised by °F and called “degrees Fahrenheit”). On this scale, the freezing point of liquid water is 32 °F, and the boiling point of water is 212 °F.
The fundamental unit of temperature in SI is the Kelvin (K). The Kelvin temperature scale (note that the name of the scale capitalises the word Kelvin, but the unit itself is lowercase) uses units that are the equal to degrees Celsius, but the numerical scale is shifted up by 273.15 units. Note that the Kelvin scale does not use the word degrees; a temperature of 295 K is spoken of as “two hundred and ninety-five kelvin” and not “two hundred and ninety-five degrees Kelvin.”
The reason that the Kelvin scale is defined this way is that there exists a minimum possible temperature called (zero kelvin). Absolute zero is a theoretical temperature at which an object’s particles will have no heat and no movement. The Kelvin temperature scale is set so that 0 K is absolute zero, and the temperature is counted upwards from there. Normal room temperature is about 295 K.
By comparing the Kelvin, Fahrenheit and Celsius scales, a conversion between the three scales can be determined:
[latex]\scriptsize \begin{array}{l} & {}^{0}\text{C}=({}^{0}\text{F}-32)\times \displaystyle \frac{5}{9}\\ & {}^{0}\text{F}=({}^{0}\text{C}\times \text{ }\displaystyle \frac{9}{5})+32\\ & \text{K}={}^{0}\text{C}+273\end{array}[/latex]
Example 2.1
- What is 98.6 °F in degrees Celsius?
- What is 25.0 °C in degrees Fahrenheit?
Solutions
- [latex]\scriptsize {}^{\text{0}}\text{C }=\left( {98.6-32} \right)\times \displaystyle \frac{5}{9}=66.6\times \displaystyle \frac{5}{9}=37.0\text{ }{}^{0}\text{C}[/latex]
- [latex]\scriptsize {}^{0}\text{F}=\left( {25.0\times \displaystyle \frac{9}{5}} \right)+32=45.0+32=77.0\text{ }{}^{0}\text{F}[/latex]
Example 2.2
If the normal room temperature is [latex]\scriptsize 72.0\text{ }{}^\circ \text{F}[/latex], what is room temperature in degrees Celsius and kelvin?
Solution
Step 1: First convert the temperature from Fahrenheit to Celsius:
[latex]\scriptsize {}^{0}\text{C}=\left( {72.0-32} \right)\times \text{ }\displaystyle \frac{5}{9}=40.0\text{ }\times \text{ }\displaystyle \frac{5}{9}=22.2{{\text{ }}^{0}}\text{C}[/latex]
Step 2: Convert the temperature from Celsius to Kelvin:
[latex]\scriptsize \text{K = 22}\text{.2}{}^{0}\text{C + 273 = 295}\text{.2 K}[/latex]
So, room temperature is about 295 K.
Example 2.3
What is 120 K in Celsius?
Solutions
[latex]\scriptsize {}^{0}\text{C = 120 - 273 = -153 }{}^{0}\text{C}[/latex]
Exercise 2.1
- Convert the following:
- 0 0C to Kelvin
- 183 K to Celsius
- In January, the average temperature of eastern Siberia is -50 °C.
- Calculate this temperature in Kelvin.
- Of the three temperature units, which one is the SI unit for temperature?
The full solutions are at the end of the unit.
Types of heat transfer
Heat can be given off in three different processes: conduction, convection, or radiation.
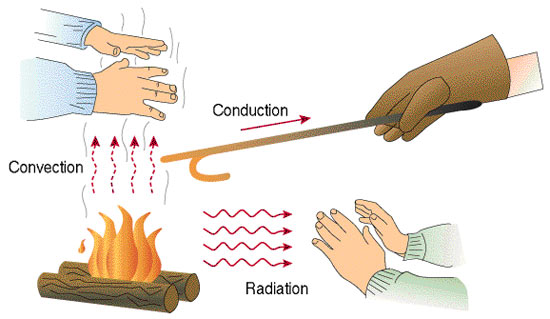
Conduction
Conduction occurs when thermal energy is transferred through the interaction of solid particles. This process often occurs when cooking where the metal pot will be heated by heat coming from the ring on the stove: the boiling of water in a metal pan causes the metal pan to warm up as well.
Conduction is the transfer of heat across a medium or objects that are in physical contact. Conduction can be imagined as the energy transfer from more energetic to less energetic particles (here, molecules) due to interaction between them.
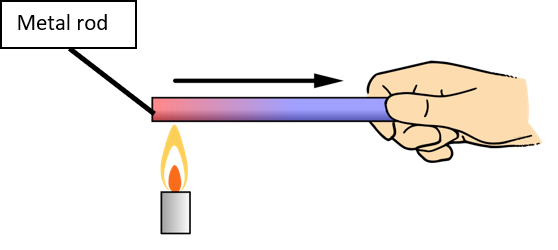
A hot pan placed on a burner burns your hand if you touch it because conduction of heat takes place between the heated pan and your hand.
Convection
Convection usually takes place in gases or liquids in which the transfer of thermal energy is based on differences in heat. Convection is the transfer of heat from a fluid or gas to a solid surface or within a fluid.
A is the best example of this mode of heat transfer. When a cast iron skillet containing water is placed on a burner, convection currents are formed in the water. Warmer water, which is less dense moves up while the colder water (more dense), sinks down.
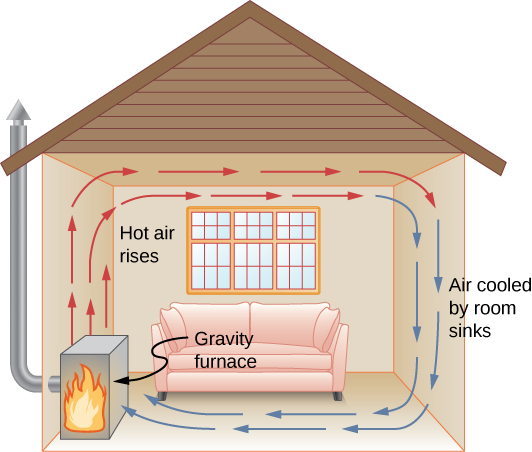
The convection currents in a room with a fireplace or heater are shown in Figure 6. The fireplace draws cool air in at the bottom, which is warmed by the flames. The hot air rises from the top keeping the room warm.
There are two types of convection:
- natural convection: this occurs due to a density difference caused by a present between a fluid and a surface
- forced convection: this is externally generated, for example by fans, pumps, and compressors, which are used to set the fluid or gas in motion.
Radiation
Radiation is the transfer of thermal energy through space and is responsible for the sunlight that fuels the Earth. You can feel the heat transfer from the sun on a sunny day.
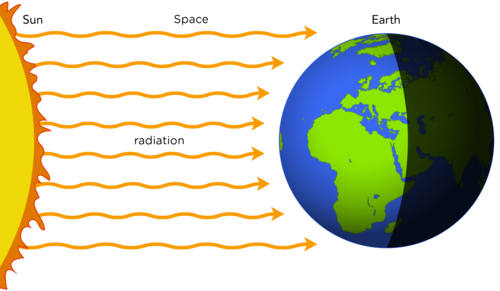
The space between Earth and the Sun is largely empty, so the sun warms us without any possibility of heat transfer by convection or conduction.
Similarly, you can sometimes tell that an oven being used for cooking is hot without touching its door or looking inside – the air near it may feel warm you as you walk by. In these examples, heat is transferred by radiation. The hot entity emits that are absorbed by the skin. No medium is required for electromagnetic waves to be generated.
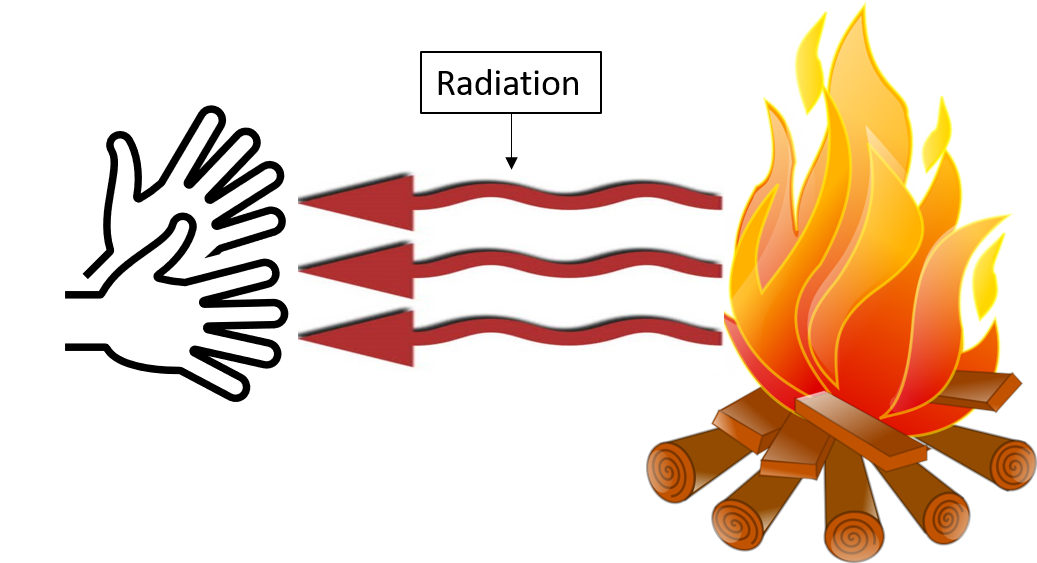
Most of the heat transfer from this fire in Figure 8 to the observers occurs through a type of radiation, called . Infrared radiation is invisible to the naked eye, and the wavelengths are longer than visible red light. A grill cooks food by radiation. A toaster toasts bread by radiation.
Skin is very sensitive to infrared radiation, so you can sense the presence of a fire without looking at it directly.
Note
To consolidate your understanding of heat and heat transfer you can watch this video called The Physics of heat, by Crash Course (Duration: 9.15).
Summary
In this unit you have learnt the following:
- Heat is a type of energy.
- Heat can be measured with a thermometer on one of three scales:
- Celsius
- Kelvin
- Fahrenheit.
- Water boils at [latex]\scriptsize 100{{\text{ }}^{0}}\text{C or 373 K}[/latex] at sea level and freezes at [latex]\scriptsize 0{{\text{ }}^{0}}\text{C or 273K}[/latex].
- Absolute zero is a theoretical temperature set at [latex]\scriptsize -273{{\text{ }}^{0}}\text{C or 0 K}[/latex].
- To convert a temperature reading from Celsius to Kelvin, you add 273 to the temperature in Celsius.
- Heat can be transferred by:
- conduction
- convection
- radiation.
Unit 2: Assessment
Suggested time to complete: 20 minutes
- Convert the following to Kelvin:
- [latex]\scriptsize 27\text{ }{}^{0}\text{C}[/latex]
- [latex]\scriptsize -31.2\text{ }{}^{0}\text{C}[/latex]
- Convert the following to Celsius:
- [latex]\scriptsize 63.2\text{ K}[/latex]
- [latex]\scriptsize 568.32\text{ K}[/latex]
- What is the coldest temperature possible? Choose the correct option.
- Total zero
- Freezing point
- Complete zero
- Absolute freezing
- Absolute zero
- Gallium is a metal that can melt in your hand at [latex]\scriptsize 302.93\text{ K}[/latex]. What is the temperature in Celsius?
- What does the temperature scale on a thermometer measure?
- In conduction, heat moves from something hot to ______________________.
- When water is boiling in a pot (refer to Figure 4 if necessary):
- Explain how a convection current is formed.
- Which other method of heat transfer is needed to heat the pot?
- Explain how the method referred to in b, and convection cause the water in the pot to warm up.
The full solutions are at the end of the unit.
Unit 2: Solutions
Exercise 2.1
- .
- [latex]\scriptsize \text{K}=0{}^{0}\text{C}+273=273\text{ K}[/latex]
- [latex]\scriptsize {}^{0}\text{C}=183\text{ K}-273=-90{}^{\text{0}}\text{C}[/latex]
- .
- [latex]\scriptsize \text{K}=-50\text{ }{}^{0}\text{C}+273=223\text{ K}[/latex]
- Kelvin
Unit 2: Assessment
- .
- [latex]\scriptsize \text{K}=27\text{ }{}^{0}\text{C + 273 = 300 K}[/latex]
- [latex]\scriptsize \text{K}=-31.2\text{ }{}^{0}\text{C + 273 = 241}\text{.8 K}[/latex]
- .
- [latex]\scriptsize {}^{0}\text{C}=63.2\text{ K - 273 = -209}\text{.8 }{}^{0}\text{C}[/latex]
- [latex]\scriptsize {}^{0}\text{C}=568.32\text{ K - 273 = 295}\text{.32 }{}^{0}\text{C}[/latex]
- e. Absolute zero
- [latex]\scriptsize {}^{0}\text{C}=302.93\text{ K - 273 = 29}\text{.93}{}^{0}\text{C}[/latex]
- The scale measures the average kinetic energy of the particles in the object.
- Something cold.
- When water is boiling in a pot (refer to Figure 4 if necessary):
- A convection current is formed when the water particles are heated and rise, then sink when they have cooled, to be heated again so they can rise again.
- Conduction.
- Conduction is needed to heat the pot. The stove burner will transfer thermal energy via conduction to the pot. As the pot warms, the pot will heat up the water by convection.
Media Attributions
- Fig 1 © Libre texts is licensed under a CC BY-SA (Attribution ShareAlike) license
- Fig 2 © โดย Kmecfiunit - งานของตัว is licensed under a CC BY-NC (Attribution NonCommercial) license
- Fig 3 © MikeRun, is licensed under a CC BY-SA (Attribution ShareAlike) license
- Fig 4 © Oni Lukos is licensed under a CC BY-SA (Attribution ShareAlike) license
- Fig 5 © Opentextbc is licensed under a CC BY (Attribution) license
- Fig 6 © Siyavula is licensed under a CC BY-ND (Attribution NoDerivatives) license
- Fig 7 © DHET is licensed under a CC BY (Attribution) license
the flow of thermal energy due to differences in temperature
the energy within a system
a measure of the kinetic energy that particles have
the total kinetic energy within a given system
the movement of thermal energy between interacting solid objects
the movement of thermal energy in liquids and gases because of a difference in temperature
dangerous levels of energy from charged particles
a theoretical temperature of exactly $latex \displaystyle -273.15\text{ }{}^\circ \text{C/ 0 K}$ characterised by the absence of heat and movement
a vertical flow of a gas or liquid moved by thermal energy
a number or scale that shows the heat energy in particles and in which direction and at what rate the temperature changes the most rapidly around a particular location
waves that are created because of vibrations between an electric field and a magnetic field
invisible electromagnetic waves, which transfer heat through radiation
