Chemical change: Identify, describe, and apply principles of chemical reactions (electrolytes)
Unit 1: Principles of chemical reactions
Emma Harrage
Unit outcomes
By the end of this unit you will be able to:
- Give a definition of an electrolyte and identify them.
- Identify salts which are sources of electrolytes.
- Determine their solubility by how much electricity they conduct.
- Identify acids which are sources of electrolytes.
- Identify the effects of ions in an aqueous solution.
What you should know:
Before you start this unit, make sure you can:
- Confidently complete all the work in Subject outcome 5.4 Unit 2: Elements, mixtures, and compounds.
- Confidently complete all the work in Subject outcome 6.2 Unit 1: Chemical and physical change.
- Confidently complete all the work in Subject outcome 6.2 Unit 2: Separating mixtures.
Introduction
Some chemical substances can conduct electricity when they are solid like metals. Other chemical substances can conduct electricity when they have been dissolved in a liquid. For a substance to conduct electricity, it must contain charged particles that are free to move. Metals, for example, conduct electricity because the valence electrons are free to move.
Solutions can conduct electricity if they contain ions, as these are charged particles and they are free to move in the solution. These substances are called electrolytes. Examples of electrolytes include acids, bases, and salt solutions.
Activity 1.1: Dissolving compounds
Time required: 10 minutes
What you need:
- a computer with JAVA installed
What to do:
- Open the Sugar and Salt Solutions simulation.
- On the top left-hand corner, press the ‘micro’ tab.
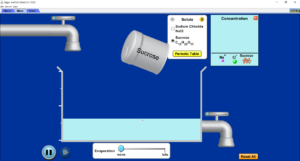
- Select sucrose as the solute, and then add sucrose to the water by shaking (clicking and dragging) the container over the water.
- What do you notice about the sucrose molecules in the water?
- Press the reset all button.
- Change the solute to sodium chloride and shake it into the solvent.
- What do you notice about the sodium chloride? How does this compare to the sucrose?
- Press the tab which says water. Add the sodium chloride.
What did you find?
Sucrose is a covalently bonded substance. The molecules are neutral. When added to water, the molecules move around in the water, but they are still neutral. Sucrose solution will not conduct electricity and is therefore not an electrolyte.
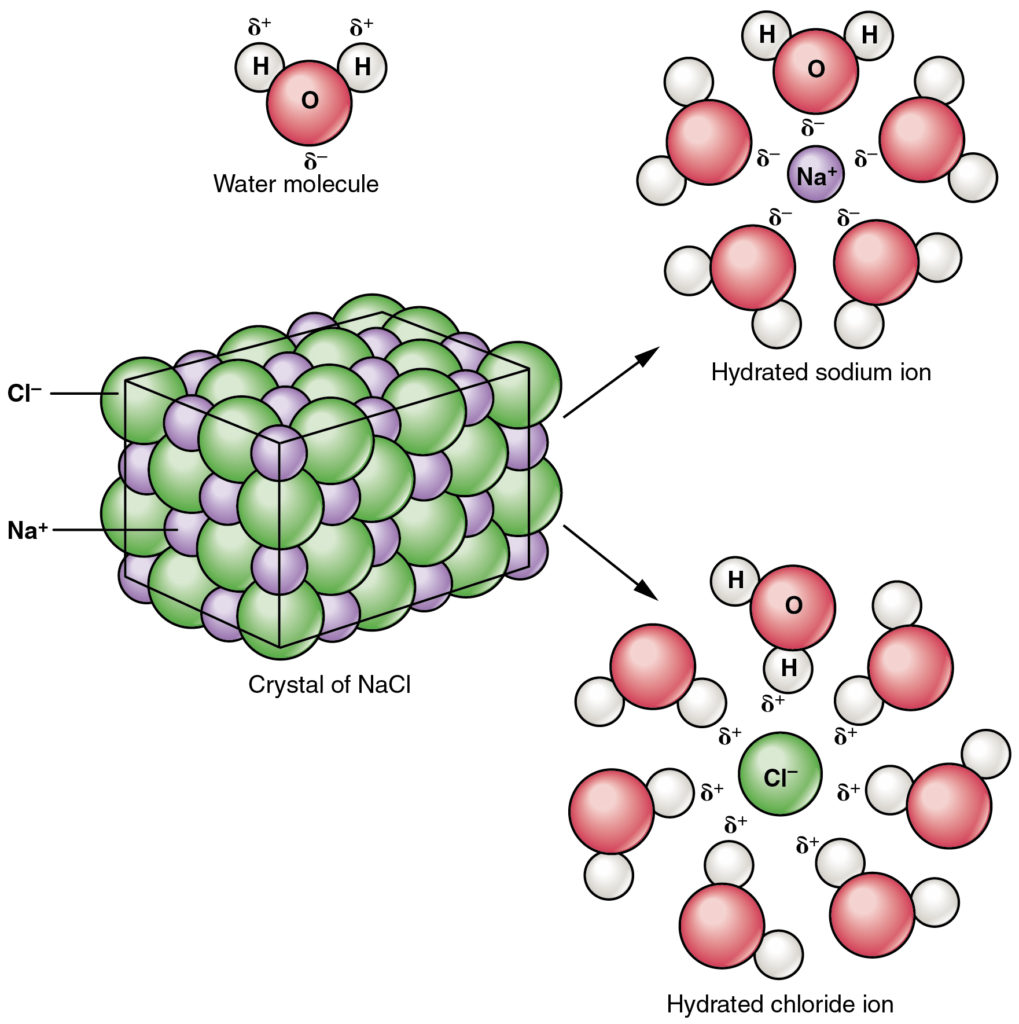
Sodium chloride has ionic bonds. In the solid phase it is made up of sodium ions (Na+) and chloride ions (Cl–) tightly packed together in a crystal lattice. When a NaCl solid dissolves in water the separate and are free to move around in the solution. This process is called . Because the ions are now free to move, the solution can conduct electricity. Sodium chloride solution is therefore an .
Electrolytes
Electrolytes are substances that contain free ions and behave as electrically conductive media. As we learnt in Activity 1.1, sodium chloride solution is an electrolyte because sodium chloride dissolves in water leaving the ions free to move around. So, the ions dissociate from one another.
The equation for the dissociation is:
[latex]\scriptsize \text{Sodium chloride}\to \text{sodium ions + chloride ions}[/latex]
[latex]\scriptsize \text{NaC}{{\text{l}}_{\text{(s)}}}\to \text{Na}_{\text{(aq)}}^{\text{+}}\text{+Cl}_{\text{(aq)}}^{\text{-}}[/latex]
When sodium metal bonds ionicly to chlorine, the sodium atoms lose electrons and form positively charged (Na+) and the chlorine atoms gain electrons to form negative (Cl–).
We say that of a substance has occurred when a substance dissociates or . Dissolving is a physical change that takes place. It can be reversed by evaporating off the water.
Most (salts) will dissociate in water. The metal ions of the are always positively charged (cations) and the non-metals ions are negatively charged (anions).
Some salts contain groups of atoms that are covalently bonded together and behave as a single unit. They can be positively or negatively charged and are called . The most common ones are listed below with their name and formula:
Ammonium: [latex]\scriptsize \text{NH}_{4}^{+}[/latex]
Nitrate: [latex]\scriptsize \text{NO}_{3}^{-}[/latex]
Carbonate: [latex]\scriptsize \text{CO}_{3}^{2-}[/latex]
Oxide: [latex]\scriptsize {{\text{O}}^{\text{-2}}}[/latex]
Permanganate: [latex]\scriptsize \text{MnO}_{4}^{-}[/latex]
Phosphate: [latex]\scriptsize \text{PO}_{4}^{3-}[/latex]
Sulphate: [latex]\scriptsize \text{SO}_{4}^{2-}[/latex]
Hydroxide: [latex]\scriptsize \text{O}{{\text{H}}^{-}}[/latex]
Hydronium: [latex]\scriptsize {{\text{H}}_{\text{3}}}{{\text{O}}^{\text{+}}}[/latex]
Acids as electrolytes
Acids will dissociate in water and form electrolytes. Weak acids form weak electrolytes and strong acids will form strong electrolytes.
For example, carbonic acid is a weak acid:
[latex]\scriptsize {{\text{H}}_{\text{2}}}\text{C}{{\text{O}}_{\text{3(aq)}}}\rightleftharpoons \text{H}_{\text{(aq)}}^{\text{+}}\text{+HCO}_{3(aq)}^{-}[/latex]
Note
The [latex]\scriptsize \rightleftharpoons[/latex] arrows indicate that the reaction is reversible.
A strong acid, for example hydrochloric acid, will dissociate easily and readily form hydronium ions ([latex]\scriptsize {{\text{H}}_{\text{3}}}{{\text{0}}^{\text{+}}}[/latex]). These are strong electrolytes.
[latex]\scriptsize \text{HC}{{\text{l}}_{(aq)}}+{{\text{H}}_{2}}{{\text{O}}_{(l)}}\to {{\text{H}}_{3}}\text{O}_{(aq)}^{+}+\text{Cl}_{(aq)}^{-}[/latex]
Chemical equations for dissociation
Chemical equations are written to show the amounts of substances involved and to show the compounds formed because of a chemical change.
Ionic equations such as: [latex]\scriptsize \text{NaC}{{\text{l}}_{\text{(s)}}}\to \text{Na}_{\text{(aq)}}^{\text{+}}\text{+Cl}_{\text{(aq)}}^{\text{-}}[/latex] are written to show the dissociation of the ionic compound in water. The + and – symbols show what charge the ions have when in an aqueous solution. The bracketed subscript (s) are state symbols; they show what state the compound or ions are in.
(s) = solid, (l) = liquid, (g )= gas, (aq) = aqueous
The dissolution of potassium sulfate into potassium and sulfate ions is shown below as another example:
[latex]\scriptsize \text{Potassium sulfate}\to \text{potassium ions + sulfate ions}[/latex]
[latex]\scriptsize {{\text{K}}_{\text{2}}}\text{S}{{\text{O}}_{\text{4(s)}}}\to \text{2K}_{\text{(aq)}}^{\text{+}}\text{+SO}_{\text{4(aq)}}^{\text{-2}}[/latex]
Ionisation
Molecular substances (covalent compounds) may also dissolve, but most will not form ions. One example is glucose:
[latex]\scriptsize \text{Glucose}\to \text{Glucose}[/latex]
[latex]\scriptsize {{\text{C}}_{\text{6}}}{{\text{H}}_{\text{12}}}{{\text{O}}_{\text{6(s)}}}\to {{\text{C}}_{\text{6}}}{{\text{H}}_{\text{12}}}{{\text{O}}_{\text{6(aq)}}}[/latex]
These solutions will not conduct electricity because there are no free-moving charged particles (ions). They are not electrolytes.
Some covalently bonded substances, however, will form ions when they dissolve by reacting with water molecules. This process is called . Hydrogen chloride, for example, can ionise to form hydrogen and chloride ions.
[latex]\scriptsize \text{hydrogen chloride+water}\to \text{hydronium ions + chloride ions}[/latex]
[latex]\scriptsize \text{HC}{{\text{l}}_{\text{(g)}}}\text{+}{{\text{H}}_{\text{2}}}{{\text{O}}_{\text{(l)}}}\to {{\text{H}}_{\text{3}}}\text{O}_{\text{(aq)}}^{\text{+}}\text{+Cl}_{\text{(aq)}}^{\text{-}}[/latex]
This solution will conduct electricity because there are now free-moving ions. It is an electrolyte.
[latex]\scriptsize \text{HC}{{\text{l}}_{(aq)}}+{{\text{H}}_{2}}{{\text{O}}_{(l)}}\to {{\text{H}}_{3}}\text{O}_{(aq)}^{+}+\text{Cl}_{(aq)}^{-}[/latex]
Below is an example of how to work with dissociation equations.
Example 1
- Write the chemical equation that represents the dissociation of the compound potassium bromide. ([latex]\scriptsize \text{KB}{\text{r}}[/latex])
- Write the chemical equation that represents the dissociation of the sodium sulphate [latex]\scriptsize \text{(N}{{\text{a}}_{\text{2}}}\text{S}{{\text{O}}_{\text{4}}}\text{)}[/latex].
Solutions:
- [latex]\scriptsize \text{KB}{\text{r}}[/latex] is an ionic compound and will completely dissociate when it is dissolved into water:
[latex]\scriptsize \text{KB}{{\text{r}}_{\text{(s)}}}\to \text{K}_{\text{(aq)}}^{\text{+}}\text{+Br}_{\text{(aq)}}^{\text{-}}[/latex]
- The two sodium ions will dissociate from the compound and go their own way, but the sulfate ion will stay together.
The dissolving equation is therefore [latex]\scriptsize \text{N}{{\text{a}}_{\text{2}}}\text{S}{{\text{O}}_{\text{4(s)}}}\to \text{ 2Na}_{(aq)}^{+}\text{ + SO}_{4(aq)}^{2-}[/latex]
Exercise 1.1
- For each of the following, say whether the substance is ionic or molecular.
- potassium nitrate ([latex]\scriptsize \text{KN}{{\text{O}}_{\text{3}}}[/latex])
- ethanol ([latex]\scriptsize {{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}\text{OH}[/latex])
- sucrose ([latex]\scriptsize {{\text{C}}_{\text{12}}}{{\text{H}}_{\text{22}}}{{\text{O}}_{\text{11}}}[/latex])
- sodium bromide ([latex]\scriptsize \text{NaBr}[/latex])
- Sodium hydroxide will dissociate in water to form sodium and hydroxide (OH) ions.
- Identify the:
- cations
- anions
- Write an ionic equation to show the dissociation. Do not forget to include state symbols.
- Identify the:
- Write dissociation equations for the following:
- iron sulfate ([latex]\scriptsize \text{FeS}{{\text{O}}_{\text{4(s)}}}[/latex])
- zinc chloride ([latex]\scriptsize \text{ZnC}{{\text{l}}_{\text{2(s)}}}[/latex])
- sodium hydroxide ([latex]\scriptsize \text{NaO}{{\text{H}}_{\text{(s)}}}[/latex])
The full solutions are at the end of the unit.
Electrolytes and conductivity
A strong electrolyte is one where many ions are in high concentration in the solution and a weak electrolyte is one where ions are in low concentration. Strong electrolytes are good conductors of electricity and weak electrolytes are weak conductors of electricity. Non-electrolytes do not conduct electricity at all.
Conductivity in aqueous solutions is a measure of the ability of water to conduct an electric current. The more ions there are in the solution, the higher its conductivity. The more ions there are in solution, the stronger the electrolyte.
Factors that affect the conductivity of electrolytes
The conductivity of an electrolyte is affected by the following factors:
- The concentration of ions in a solution. The higher the concentration of ions in a solution, the higher its conductivity will be.
- The type of substance that dissolves in water. Whether a material is a strong electrolyte (for example potassium nitrate, ([latex]\scriptsize \text{KN}{{\text{O}}_{\text{3}}}[/latex]), a weak electrolyte (for example acetic acid, [latex]\scriptsize \text{C}{{\text{H}}_{\text{3}}}\text{COOH}[/latex]) or a non-electrolyte (for example sugar, alcohol or oil) will affect the conductivity of water because the concentration of ions in a solution will be different in each case. Strong electrolytes form free-moving ions easily, weak electrolytes do not form ions easily and non-electrolytes do not form ions in a solution.
- Temperature. The warmer the solution, the higher the solubility of the material being dissolved and therefore the higher the conductivity as well.
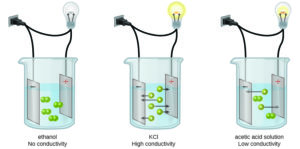
Solutions of non-electrolytes such as ethanol do not contain dissolved ions and cannot conduct electricity. Solutions of electrolytes contain ions that permit the passage of electricity. The conductivity of an electrolyte solution is related to the strength, concentration, and temperature of the electrolyte.
Activity 1.2: Conduction of electricity in a solution
Time required: 10 minutes
What you need:
- a computer with JAVA installed
What to do:
- Open the Sugar and Salt Solutions simulation.
- On the top left-hand corner, press the ‘macro’ tab.
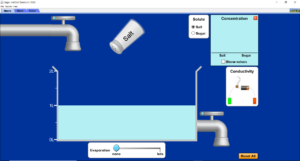
- Drag the conductivity apparatus into the water.
- Add salt to the water by shaking the salt cellar with your mouse.
- What do you notice about the brightness of the bulb?
- Press the reset all button.
- Add the conductivity apparatus, select sugar as the solute add sugar to the water with your mouse.
- What do you notice about how the sugar effects the bulb? How does this compare to the salt (sodium chloride)?
What did you find?
The salt (sodium chloride) dissociates into sodium and chlorine ions when it dissolves in the water, and the bulb lights up. The more sodium chloride you add, the brighter the bulb becomes. This is because an electrolyte can conduct electricity. If you were to replace the bulb with an ammeter, you would be able to read the current flowing through the solution.
The bulb did not light up when using the sugar (sucrose) solution. It is therefore not an electrolyte and does not conduct electricity. Sucrose is a covalent compound that does not ionise in a solution.
Note
Remember:
- For electricity to flow, there needs to be a movement of charged particles, e.g. ions.
- Distilled water, oil and alcohol do not conduct a current because they are covalent compounds and do not ionise in a solution.
- The concentration of the ions in a solution affects the amount of current flowing through the solution. The higher the concentration the higher the current, and vica versa.
Exercise 1.2
Use the information in the table below to answer the following questions:
| Salt | Amount of salt dissolved g/l | Current (A) |
| Sodium chloride | [latex]\scriptsize 0.5[/latex] | [latex]\scriptsize 0.15[/latex] |
| Sodium chloride | [latex]\scriptsize 1.0[/latex] | [latex]\scriptsize 0.36[/latex] |
| Potassium nitrate | [latex]\scriptsize 0.5[/latex] | [latex]\scriptsize 0.2[/latex] |
| Potassium nitrate | [latex]\scriptsize 1.0[/latex] | [latex]\scriptsize 0.45[/latex] |
| Hydrogen chloride | [latex]\scriptsize 0.5[/latex] | [latex]\scriptsize 0.05[/latex] |
| Hydrogen chloride | [latex]\scriptsize 1.0[/latex] | [latex]\scriptsize 0.09[/latex] |
Assuming the temperature of the water is at 15 oC for all the above.
- Use the information to draw a bar graph of the results.
- Which substance is the strongest electrolyte? Explain your answer.
- Explain why the currents recorded for both solutions of hydrogen chloride are less than that of sodium chloride.
- List two factors which could be changed to increase the conductivity of a solution of sodium chloride.
The full solutions are at the end of the unit.
Displacement of ions in an aqueous solution
When a solid metal is placed in a salt solution, a reaction will happen if the solid metal is more reactive than the metal ions in the salt solution. The positive metal ions in the solution gain electrons to become a solid, and the solid metal atoms lose electrons and go into the solution as ions. This process is called ; one metal element has taken the place of another (less reactive) metal element in the solution. In this process a solid metal corrodes away (the more reactive metal) and the metal that had ions in the solution forms a solid.
Therefore, salt solutions can be described as corrosive.
Iron corrodes in the presence of water and oxygen and this type of corrosion is called rusting. Rusting requires both oxygen and water, and it is usually sped up by acids, strains in the iron, contact with less-active metals, and the presence of rust itself. Iron objects will rust very quickly in the presence of salt water.
The equation for this is:
[latex]\scriptsize \text{Iron+hydrogen+oxygen}\to \text{Iron oxide + water}[/latex]

Rust is porous and is a flaky red solid. Once an iron object starts rusting, measures need to be taken to remove the rust and prevent further corrosion.
Aluminium metal reacts with oxygen in the air to form aluminium oxide. This kind of corrosion causes a protective layer to form on the aluminium and prevents any further corrosion.
[latex]\scriptsize \text{aluminium }\!\!~\!\!\text{ + }\!\!~\!\!\text{ oxygen }\!\!~\!\!\text{ }\!\!~\!\!\text{ }\to \text{aluminium oxide}[/latex]
The same process also occurs to copper. The Statue of Liberty in New York City has a copper skin and was a brown colour when it was first delivered to the USA in 1885. It now a green colour, due to the corrosion of the copper skin in the presence of carbon dioxide and water. This has formed a protective layer of copper carbonate on the statue.

Summary
In this unit you have learnt the following:
- Dissociation is a general process in which ionic compounds separate into smaller ions when dissolved in water.
- Conductivity is a measure of a solution’s ability to conduct an electric current.
- An electrolyte is a substance that contains free ions and is therefore able to conduct an electric current.
- Electrolytes can be divided into strong and weak electrolytes, based on the concentration of ions in the solution.
- A non-electrolyte cannot conduct an electric current because it does not contain free ions.
- The type of substance, the concentration of ions and the temperature of the solution affect the conductivity of an electrolyte.
Unit 1: Assessment
Suggested time to complete: 20 minutes
- Give a definition for the following terms:
- an electrolyte
- a strong electrolyte
- a weak electrolyte
- What factors affect the conductivity of ions in water?
- For each of the following substances state whether they are molecular or ionic.
- methane ([latex]\scriptsize \text{C}{{\text{H}}_{\text{4}}}[/latex])
- potassium bromide ([latex]\scriptsize \text{KBr}[/latex])
- carbon dioxide ([latex]\scriptsize \text{C}{{\text{O}}_{\text{2}}}[/latex])
- hexane ([latex]\scriptsize {{\text{C}}_{\text{6}}}{{\text{H}}_{\text{14}}}[/latex])
- lithium fluoride ([latex]\scriptsize \text{LiF}[/latex])
- magnesium chloride ([latex]\scriptsize \text{MgCl}[/latex])
- Write equations to show how each of the following ionic compounds dissociate in water.
- sodium sulphate ([latex]\scriptsize \text{N}{{\text{a}}_{2}}\text{S}{{\text{O}}_{4}}[/latex])
- potassium permanganate ([latex]\scriptsize \text{KMn}{{\text{O}}_{\text{4}}}[/latex])
- sodium phosphate ([latex]\scriptsize \text{N}{{\text{a}}_{\text{3}}}\text{P}{{\text{O}}_{\text{4}}}[/latex])
The full solutions are at the end of the unit.
Unit 1: Solutions
Exercise 1.1
- .
- potassium nitrate ([latex]\scriptsize \text{KN}{{\text{O}}_{\text{3}}}[/latex]) is an ionic compound because there is a metal bonded to non-metals
- ethanol ([latex]\scriptsize {{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}\text{OH}[/latex]) is a molecular compound because there are no metals in the compound only non-metals
- sucrose ([latex]\scriptsize {{\text{C}}_{\text{12}}}{{\text{H}}_{\text{22}}}{{\text{O}}_{\text{11}}}[/latex]) is a molecular compound because there are no metals in the compound only non-metals
- sodium bromide ([latex]\scriptsize \text{NaBr}[/latex]) is an ionic compound because there is a metal bonded to a non-metal
- .
- .
- cations – sodium ions. Sodium is a metal so it will form positively charged ions.
- anions – hydroxide ions. They are non-metals so will form negatively charged ions.
- [latex]\scriptsize \text{NaO}{{\text{H}}_{\text{(s)}}}\text{ }\to \text{Na}_{\text{(aq)}}^{\text{+}}\text{+OH}_{\text{(aq)}}^{\text{-}}[/latex] Sodium will form positively charged ions in an aqueous solution and hydroxide will form a negatively charged ion.
- .
- .
- [latex]\scriptsize \text{FeS}{{\text{O}}_{\text{4(s)}}}\text{ }\to \text{ Fe}_{(aq)}^{2+}\text{+ SO}_{4(aq)}^{2-}[/latex]
- [latex]\scriptsize \text{ZnCl}_{\text{(s)}}^{\text{2}}\text{ }\to \text{Zn}_{\text{(aq)}}^{\text{2+}}\text{ + 2Cl}_{\text{(aq)}}^{\text{-}}[/latex]
- [latex]\scriptsize \text{NaO}{{\text{H}}_{(s)}}\text{ }\to \text{ Na}_{(aq)}^{+}\text{ + OH}_{(aq)}^{-}[/latex]
Exercise 1.2
- .
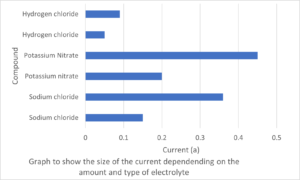
- Potassium nitrate is the strongest electrolyte because when 1g/l is dissolved it produces the highest current.
- Hydrogen chloride is a weak electrolyte compared to sodium chloride.
- – Increase the concentration of sodium chloride in the solution.
– increase the temperature of the aqueous solution.
Unit 1: Assessment
- .
- an electrolyte – a solution that will conduct electricity
- a strong electrolyte – a solution with a high centration of ions and will therefore conduct electricity well
- a weak electrolyte – a solution with a low concentration of ions and will therefore not conduct electricity very well
- Temperature, concentration, and type of substance
- .
- molecular
- ionic
- molecular
- molecular
- ionic
- ionic
- .
- [latex]\scriptsize \text{N}{{\text{a}}_{2}}\text{S}{{\text{O}}_{4}}_{\text{(s)}}\to \operatorname{Na}_{(aq)}^{+}+SO_{4(aq)}^{-2}[/latex]
- [latex]\scriptsize \text{KMn}{{\text{O}}_{\text{4}}}_{\text{(s)}}\to K_{(aq)}^{+}+MnO_{4(aq)}^{-}[/latex]
- [latex]\scriptsize \text{N}{{\text{a}}_{\text{3}}}\text{P}{{\text{O}}_{\text{4}}}_{\text{(s)}}\to \text{3N}{{\text{a}}^{\text{+}}}_{\text{(aq)}}\text{+PO}_{\text{4(aq)}}^{\text{-3}}[/latex]
Media Attributions
- Phet simulation 1 © Phet is licensed under a CC BY (Attribution) license
- dissociation of sodium chloride in water © Rice University is licensed under a CC BY (Attribution) license
- electolytes conducting electricity © Libretext is licensed under a CC BY-NC (Attribution NonCommercial) license
- Phet simulation 3 © Phet is licensed under a CC BY (Attribution) license
- Rusty chain © Moshe Harosh is licensed under a CC0 (Creative Commons Zero) license
- Statofliberty is licensed under a CC BY (Attribution) license
- Graph to show the current vs electrolyte © Department of Higher Education and Training is licensed under a CC BY (Attribution) license
positively or negatively charged particles
the process in which ionic compounds separate into smaller ions usually in a reversible manner, when dissolved in water
a substance that contains free ions and behaves as an electrically conductive medium
positively charged ions
a positively charged particle
process where ionic crystals break up into ions in water
separates when placed in water; the ions in an ionic compound separate out from the crystal lattice and move freely
compounds formed between a metal and a non-metal
a compound formed from a metal and a non-metal, such as sodium chloride (NaCl), or a metal and a polyatomic ion, such as sodium hydroxide (NaOH)
ions composed of a number of atoms that are covalently bonded.
the process when a covalently bonded compound reacts with water to form ions in a solution
a chemical reaction where one element is replaced by another in a compound
