Matter and materials: Identify, describe and apply properties of the periodic table
Unit 1: The periodic table
Emma Harrage
Unit outcomes
By the end of this unit you will be able to:
- Describe the arrangement of the periodic table according to an element’s atomic number.
- Identify that elements are in vertical groups according to similar chemical properties.
- Identify that elements are arranged in horizontal periods according to increasing atomic number.
- Recall that periodicity means the arrangement on the periodic table of elements because of their:
- atomic radius
- electronegativity
- ionisation energy.
What you should know:
Before you start this unit, make sure you can:
Confidently complete all the work in Subject outcome 5.2, Unit 1: The atom.
Confidently complete all the work in Subject outcome 5.2, Unit 2: Atomic number and atomic mass.
Introduction
In this unit you will learn to recognise the arrangement of atoms on the periodic table according to atomic number, groups, periods, and periodicity.
Elements are laid out vertically across the periodic table according to their atomic number (the number of protons they have). They are grouped horizontally according to their similar properties and periods because they have the same number of electron shells.
Introduction to the periodic table
The of the elements is a method of showing the chemical elements in a table with the elements arranged in order of increasing . The layout of the periodic table that we know today can mostly be attributed to a Russian chemist named Dimitri Mendeleev. Mendeleev designed the table in 1869 in such a way that recurring (“periodic”) trends or patterns in the properties of the elements are apparent. Using the trends he observed, he left gaps for those elements that he thought were “missing”. He also predicted the properties that he thought the missing elements would have when they were discovered. Many of these elements were indeed discovered and Mendeleev’s predictions were proved to be correct.
To show the recurring properties that he had observed, Mendeleev used a series of new rows in his table so that elements with similar properties were in the same vertical columns, called . Each row was referred to as a .
Note
You can download an interactive periodic table app from The Royal Society of Chemistry for either Android or Apple iOS.
Or use this interactive link online.
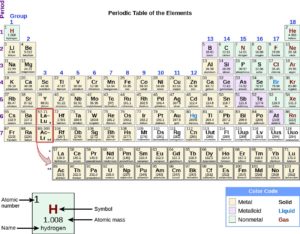
Each element has an abbreviated form of its name. Most of the elements’ abbreviations are either the first letter of their name and may include the second letter, which is always written in lower case, for example:
| oxygen | O |
| carbon | C |
| lithium | Li |
| calcium | Ca |
| silicon | Si |
| sulfur | S |
| helium | He |
Some of the abbreviations are not abbreviations of the English element name, but of the Latin or Greek element name, because they were discovered a long time ago, for example:
| Latin Name | English Name | Chemical Symbol |
| Plumbum | Lead | Pb |
| Aurum | Gold | Au |
| Ferrum | Iron | Fe |
| Argentum | Silver | Ag |
| Natrium | Silver | Na |
Did you know?
Water pipes used to be made of lead until it was discovered that lead is soluble in water and causes poor cognitive development. Plumbers are called plumbers because of lead’s Latin name plumbum.
Note
For a further introduction to the periodic table you can watch this video:
Socratia: Introduction to the Periodic Table (Duration: 9.05)
or this video from Fuse Schools: How does the periodic table work (Duration: 4.30). It is part of an excellent playlist.
Layout of the periodic table
A group is a vertical column in the periodic table and is the most important way of classifying the elements. If you look at a periodic table, you will see that at the top of each column, or group, there is a number. The groups are numbered from left to right starting with 1 and ending with 18.
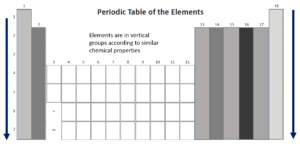
Elements were placed in groups (columns) according to an increasing atomic number and in periods (rows) because they have similar chemical properties. The characteristics of each group are mostly determined by the of the atoms of the elements in the group. A period is a horizontal row in the periodic table of the elements. The periods are labelled from top to bottom, starting with 1 and ending with 7.
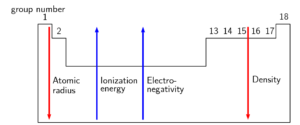
Atomic radius, electronegativity, and ionisation energy
is the energy needed to remove one electron from an atom in the gas phase. The ionisation energy is different for each element. The is the energy needed to remove the first electron from an atom.
is the tendency of atoms to attract electrons. The electronegativity of the elements starts from about 0.7 (francium (Fr)) and goes up to 4 (fluorine (F)).
The is a measure of the size of an atom.
Periods
Let’s take a closer look at the periods in the periodic table.
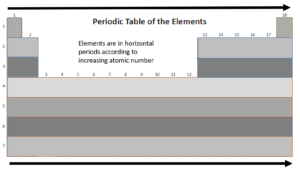
As you travel from left to right across a period, electronegativity and ionisation energy increase, and the atomic radius of an element decreases.

Let’s look at period 3 of the periodic table:
| Group | [latex]\scriptsize 1[/latex] | [latex]\scriptsize 2[/latex] | [latex]\scriptsize 13[/latex] | [latex]\scriptsize 14[/latex] | [latex]\scriptsize 15[/latex] | [latex]\scriptsize 16[/latex] | [latex]\scriptsize 17[/latex] |
| Element | Sodium | Magnesium | Aluminium | Silicon | Phosphorus | Sulfur | Chlorine |
| Atomic radius | Decreases across a period. | ||||||
| First ionisation energy | The general trend is an increase across the period. | ||||||
| Electronegativity | Increases across the period. | ||||||
| Melting and boiling point | Increases to silicon and then decreases to argon. | ||||||
| Electrical conductivity | Increases from sodium to aluminium. Silicon is a semi-conductor. The rest are insulators. | ||||||
Groups
Let’s take a closer look at the groups in the periodic table.
As you move down a group, the atomic radius and density of the element increase but the electronegativity and ionisation energy decrease.
Let’s look at Group 1 of the periodic table:
| Period | [latex]\scriptsize 2[/latex] | [latex]\scriptsize 3[/latex] | [latex]\scriptsize 4[/latex] | [latex]\scriptsize 5[/latex] | [latex]\scriptsize 6[/latex] |
| Element | Lithium | Sodium | Potassium | Rubidium | Caesium |
| Atomic radius | Increases as you move down the group. | ||||
| First ionisation energy | Decreases as you move down the group. | ||||
| Electronegativity | Decreases as you move down the group. | ||||
| Melting and boiling points | Decrease as you move down the group. | ||||
| Density | Increases as you move down the group. | ||||
Please note that hydrogen is not included in the above table because it is a non-metal gas and not an actual member of Group 1. Francium has not been included because it is an extremely rare element.
Note
For further explanation on periods and groups you can watch this video:
Fuse Schools: Periods and groups in the periodic table (Duration: 2.53).
Exercise 1.1
- Give one word or term for each of the following:
- The energy that is needed to remove one electron from an atom
- A row on the periodic table
- A column on the periodic table
- Which of the following are denser:
- lithium or potassium?
- boron or tin?
- Atomic radius and density have a trend in common. What is it?
- The following data table gives the ionisation energy and atomic number ([latex]\scriptsize Z[/latex]) for a number of elements in the periodic table:
[latex]\scriptsize Z[/latex] Name of element Ionisation energy (kJ mol−1) [latex]\scriptsize Z[/latex] Name of element Ionisation energy (kJ mol−1) [latex]\scriptsize 1[/latex] [latex]\scriptsize 1310[/latex] [latex]\scriptsize 10[/latex] [latex]\scriptsize 2072[/latex] [latex]\scriptsize 2[/latex] [latex]\scriptsize 2360[/latex] [latex]\scriptsize 11[/latex] [latex]\scriptsize 494[/latex] [latex]\scriptsize 3[/latex] [latex]\scriptsize 517[/latex] [latex]\scriptsize 12[/latex] [latex]\scriptsize 734[/latex] [latex]\scriptsize 4[/latex] [latex]\scriptsize 895[/latex] [latex]\scriptsize 13[/latex] [latex]\scriptsize 575[/latex] [latex]\scriptsize 5[/latex] [latex]\scriptsize 797[/latex] [latex]\scriptsize 14[/latex] [latex]\scriptsize 783[/latex] [latex]\scriptsize 6[/latex] [latex]\scriptsize 1087[/latex] [latex]\scriptsize 15[/latex] [latex]\scriptsize 1051[/latex] [latex]\scriptsize 7[/latex] [latex]\scriptsize 1397[/latex] [latex]\scriptsize 16[/latex] [latex]\scriptsize 994[/latex] [latex]\scriptsize 8[/latex] [latex]\scriptsize 1307[/latex] [latex]\scriptsize 17[/latex] [latex]\scriptsize 1250[/latex] [latex]\scriptsize 9[/latex] [latex]\scriptsize 1673[/latex] [latex]\scriptsize 18[/latex] [latex]\scriptsize 1540[/latex] Copy the table into your notebook and complete the following:
- Fill in the names of the elements.
- Draw a line graph, with atomic number on the x-axis and ionisation energy on the -y axis.
- Describe any trends you can see.
The full solutions are at the end of the unit.
Summary
In this unit you have learnt the following:
- Elements are arranged in periods and groups in the periodic table. The elements are arranged from left to right according to increasing atomic number.
- A group is a column on the periodic table containing elements with similar properties.
- A period is a row on the periodic table.
- The atomic radius is a measure of the size of the atom.
- The first ionisation energy is the energy needed to remove one electron from an atom in the gas phase.
- Electronegativity is the tendency of atoms to attract electrons.
- Across a period, the ionisation energy and electronegativity increase. The atomic radius decreases across a period.
- The atomic radius and the density both increase down a group. The ionisation energy, electronegativity, and melting and boiling points all decrease down a group.
Unit 1: Assessment
Suggested time to complete: 45 minutes
- Magnesium and calcium are both found in Group 2 of the periodic table. Compare these elements in terms of the following properties and explain the differences in each case.
- Size of the atom (atomic radius)
- Electronegativity
- First ionisation energy
- Boiling point
- Compare bromine and chlorine in terms of the following properties. Explain the differences in each case.
- Atomic radius
- Electronegativity
- First ionisation energy
- Boiling point
- State the difference between a period and a group.
- Give one word or term for each of the following:
- The energy that is needed to remove one electron from an atom.
- A horizontal row on the periodic table.
- A very reactive group of elements that is missing just one electron from their outer shells.
- Look at the following table:
Element NA Mg Al Si P S Cl Ar Atomic number [latex]\scriptsize 11[/latex] [latex]\scriptsize 12[/latex] [latex]\scriptsize 13[/latex] [latex]\scriptsize 14[/latex] [latex]\scriptsize 15[/latex] [latex]\scriptsize 16[/latex] [latex]\scriptsize 17[/latex] [latex]\scriptsize 18[/latex] Density (g⋅cm−3) [latex]\scriptsize 0.97[/latex] [latex]\scriptsize 1.74[/latex] [latex]\scriptsize 2.70[/latex] [latex]\scriptsize 2.30[/latex] [latex]\scriptsize 1.82[/latex] [latex]\scriptsize 2.08[/latex] [latex]\scriptsize 3.17[/latex] [latex]\scriptsize 1.78[/latex] Melting point (℃) [latex]\scriptsize 370.9[/latex] [latex]\scriptsize 923[/latex] [latex]\scriptsize 933.5[/latex] [latex]\scriptsize 1687[/latex] [latex]\scriptsize 3173[/latex] [latex]\scriptsize 388.4[/latex] [latex]\scriptsize 171.6[/latex] [latex]\scriptsize 83.8[/latex] Boiling point (℃) [latex]\scriptsize 1156[/latex] [latex]\scriptsize 1363[/latex] [latex]\scriptsize 2792[/latex] [latex]\scriptsize 3538[/latex] [latex]\scriptsize 550[/latex] [latex]\scriptsize 717.8[/latex] [latex]\scriptsize 239.1[/latex] [latex]\scriptsize 87.3[/latex] Electronegativity [latex]\scriptsize 0.93[/latex] [latex]\scriptsize 1.31[/latex] [latex]\scriptsize 1.61[/latex] [latex]\scriptsize 1.90[/latex] [latex]\scriptsize 2.19[/latex] [latex]\scriptsize 2.58[/latex] [latex]\scriptsize 3.16[/latex] – Draw line graphs to show the patterns in the following physical properties:
- Density
- Boiling point
- Melting point
- Electronegativity
The full solutions are at the end of the unit.
Unit 1: Solutions
Exercise 1.1
- .
- First ionisation energy
- Period
- Group
- .
- Potassium
- Tin
- They both decrease as you go down a group.
- .
- .
[latex]\scriptsize Z[/latex] Name of element Ionisation energy (kJ mol−1) [latex]\scriptsize Z[/latex] Name of element Ionisation energy (kJ mol−1) [latex]\scriptsize 1[/latex] Hydrogen [latex]\scriptsize 1310[/latex] [latex]\scriptsize 10[/latex] Neon [latex]\scriptsize 2072[/latex] [latex]\scriptsize 2[/latex] Helium [latex]\scriptsize 2360[/latex] [latex]\scriptsize 11[/latex] Sodium [latex]\scriptsize 494[/latex] [latex]\scriptsize 3[/latex] Lithium [latex]\scriptsize 517[/latex] [latex]\scriptsize 12[/latex] Magnesium [latex]\scriptsize 734[/latex] [latex]\scriptsize 4[/latex] Beryllium [latex]\scriptsize 895[/latex] [latex]\scriptsize 13[/latex] Aluminium [latex]\scriptsize 575[/latex] [latex]\scriptsize 5[/latex] Boron [latex]\scriptsize 797[/latex] [latex]\scriptsize 14[/latex] Silicon [latex]\scriptsize 783[/latex] [latex]\scriptsize 6[/latex] Carbon [latex]\scriptsize 1087[/latex] [latex]\scriptsize 15[/latex] Phosphorus [latex]\scriptsize 1051[/latex] [latex]\scriptsize 7[/latex] Nitrogen [latex]\scriptsize 1397[/latex] [latex]\scriptsize 16[/latex] Sulfur [latex]\scriptsize 994[/latex] [latex]\scriptsize 8[/latex] Oxygen [latex]\scriptsize 1307[/latex] [latex]\scriptsize 17[/latex] Chlorine [latex]\scriptsize 1250[/latex] [latex]\scriptsize 9[/latex] Fluorine [latex]\scriptsize 1673[/latex] [latex]\scriptsize 18[/latex] Argon [latex]\scriptsize 1540[/latex] - .
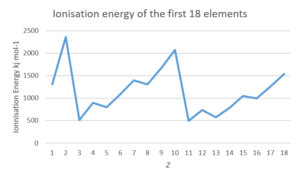
- Ionisation energy is higher for non-metals than metals. It increases as you move across a period, then decreases at the start of a new period.
- .
Unit 1: Assessment
- Calcium has a bigger atomic radius
- Calcium’s is lower
- Calcium’s is lower
- Calcium’s is lower
- .
- Chlorine’s is larger
- Chlorine’s is higher
- Chlorine’s is higher
- Chlorine’s is higher
- A period is a row and a group is a column.
- .
- First ionisation energy
- Period
- Group 17 The Halogens
- .
- .
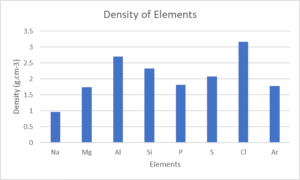
- and c.
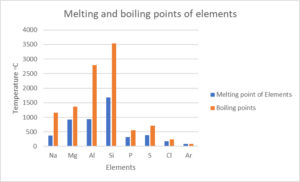
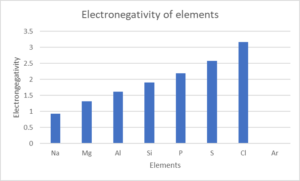
- .
Media Attributions
- Periodic Table © Openstax is licensed under a CC BY (Attribution) license
- Groups PT © DHET is licensed under a CC BY (Attribution) license
- Periods PT © DHET is licensed under a CC BY (Attribution) license
- trends on the pt © Siyavula is licensed under a CC BY (Attribution) license
- trends on the pt2 © Siyavula is licensed under a CC BY (Attribution) license
- Graph ionisation energy © DHET is licensed under a CC BY (Attribution) license
- Graph Density © DHET is licensed under a CC BY (Attribution) license
- Graph melitng and boling points © DHET is licensed under a CC BY (Attribution) license
- Graph Electronegativity © DHET is licensed under a CC BY (Attribution) license
the table of all known elements found on earth arranged in periods and groups
the number of protons in an atom
rows of elements arranged together because they have similar chemical properties
columns of elements, arranged according to increasing atomic number
the arrangement of electrons in an atom
the energy needed to remove one electron
the energy needed to remove the first electron from an atom
the tendency of an atom to attract electrons
the size of the atom
