Matter and materials: Identify, describe, and classify matter according to different macroscopic properties
Unit 1: Phases of matter
Emma Harrage
Unit outcomes
By the end of this unit you will be able to:
- Identify and describe the phases of matter
- Tell the difference between the phases of matter by their:
- Energy
- Shape
- Volume
Introduction
In this unit, you will explore the three phases of matter and then look at the properties and differences between them. You will explore their shape, volume, and kinetic energy. Let’s get started by doing an activity.
Activity 1.1: Make a model to see how particles behave
Time required: 20 minutes
What you will need:
- a rectangular box (as big as a shoebox lid,) large enough to allow free movement for five marbles
- 5 marbles (or small hard balls of the same size) – one should be different in colour from the others
- a broad flat board that can fit into the box and can close off the end of the box (see diagram in Part A), like a small ruler
- a watch or timer, for example on a cellphone
What to do:
Task 1
Place the marbles in the box on a flat surface such as a table. (Remember that your box should be large enough to allow free movement of the marbles.) Shake the box in all directions for 30 seconds, but without lifting it off the table. Then answer the questions below:
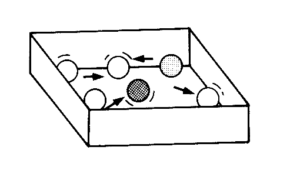
- Describe the movement of the marbles.
- Can you predict where a marble will move? Explain your answer.
- Repeat this task and watch one of the marbles. List all the things it bumps against in the 30 seconds.
We call this disorganised movement shown by the marbles random motion (disorganised movement by particles).
In task 2, we will reduce the space in the box to find out how the marbles behave in a smaller space.
Task 2
Use the broad flat board (or a ruler) to make the space inside the box half as big, as shown here. Now shake the box as before. Answer the following questions:
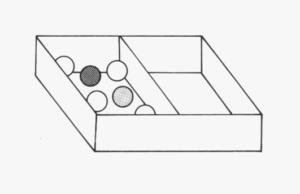
- Compare the movement of the marbles with your observations in task 1.
- Count the number of collisions the marble makes with its neighbouring marbles and with the sides of the box.
In task 3 we will decrease the space even further.
Task 3
Place your board such that the marbles are as tightly packed as possible, but do not squash them. Shake the box as before, and then answer the questions below:
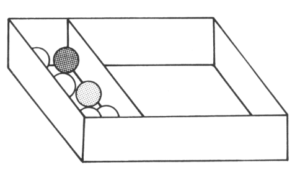
- How do the marbles move now?
- Shake the box faster.
- Can you see any difference in the motion of the marbles?
- Count the collisions of the marbles and compare the movement of the marbles now to how they moved in tasks 1 and 2 of this activity.
What did you find?
In task 1, the marbles were far apart and moved freely in a large space. This is also true of the particles in a gas. They have large spaces between them and move about freely. You would have observed, during the activity, that the particles collide with one other and with the sides of the container. There are also large spaces between the particles, on average. By ‘on average’ we mean there are large spaces most of the time except when they are colliding. Clearly, when they are colliding, they are close together. But they have great freedom of movement.
In task 2, the particles were closer together but very loosely packed and there was some room for the marbles to move. This is like the arrangement of particles in a liquid, where the particles are close together, but they can still slide over one another.
In task 3, the marbles were fixed in position and could not move about. This arrangement is similar to the arrangement of particles in solids.
Phases of matter
Matter is the name given to all the things around you. All matter is made of particles. Matter can be found in one of three phases: It can be a gas, liquid, or solid. These are called the . As you can see in Figure 1, each state of matter has particular properties which can be used to identify them.

Properties of the phases of matter
Each phase of matter has specific properties by which it can be identified, and which determine its behaviour as matter.
Solids
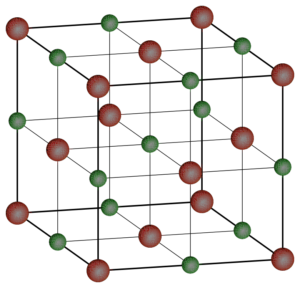
As shown in Figure 2, solids form dense 3-D lattice structures. It takes energy to break apart these structures because the particles in a solid have a strong force of attraction and this means that solids also have a high density. There is no space between the particles so you cannot compress or pour a solid. Examples of solids include gold, flour, sand, wood, diamonds, ice, and coal.
Liquids
Liquids have spaces between the particles which means they can flow past one other. You can pour a liquid. Liquids have a fixed volume and will take the shape of the bottom of any container. Liquids have a medium density because of the spaces between them, but you cannot easily compress a liquid. Examples of liquids include milk, water, oil, petrol, and ethanol.
Gases
Gases have more energy than solids and liquids. This means that they can move around in all directions, filling the whole container in which they are stored. Gases can be poured and can be compressed because there is a lot of space between the particles. Gases have a low density and have a very weak force of attraction between the particles. Examples of gases include carbon dioxide, oxygen, methane, helium, and water vapour.
Did you know?
There is a fourth state of matter called plasma. Plasma is the most common state of matter found on earth. Simply put, plasmas are very excited gases and give out light and heat which is why they are used in television sets, and in plasma torches to cut through pieces of metal. The sun is the biggest ball of plasma that we can see from Earth. Other examples are stars, lightning, and the aurorae.

Summary of the properties of the phases of matter
Here is a summary of the properties of the three phases of matter.
| Solid | Liquid | Gas |
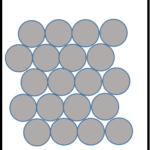 |
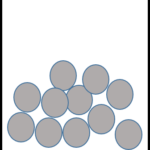 |
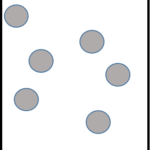 |
| Particles very close together | Particles further apart | Particles very far apart |
| Fixed lattice shape | Takes the shape of bottom of container | Takes the shape of the whole container |
| Cannot flow; particles vibrate around a fixed position | Can flow; particles can move past each other | Can flow; particles move freely in all directions |
| Fixed volume | Fixed volume | No fixed volume |
| High density | Medium density | Very low density |
| Cannot compress | Cannot compress | Can compress |
| Strong forces of attraction | Weaker forces of attraction | Very weak forces of attraction |
is a measure of how much particles have. The hotter something is, the more energy the particles have, and the colder something is, the less energy the particles have. Particles in a solid have much less energy than the particles in a gas. The particles in a bowl of ice cream are going to have much less energy than the particles in a steaming cup of tea.
Activity 1.2: Investigate the phases of matter
Time required: 15 minutes
What you will need:
- An Internet connection
What to do:
- Open this simulation.
- Click on States of Matter, or States.
- Change the atoms and molecules from neon to water.
- Change the temperature from K to °C.
- Using the slider, slowly add heat to the solid ice to about -5 °C and observe how the molecules change shape and volume and how the molecules have more kinetic energy. Can you explain why the shape, volume, and motion changes as the temperature changes?
- Heat the liquid water to about 60 °C using the slider. Can you explain why some of the molecules are ‘breaking away’ from the liquid?
- Heat the liquid to 100 °C. What happens to the number of particles that are ‘breaking away’ from the liquid?
What did you find?
As the temperature increases in Step 5, the particles will gain more energy and their movement will increase.
If the particles are in a solid state and the temperature increases, then the state of matter will change, and it will become a liquid. This will change the shape and the motion of the particles, but not necessarily the volume.
If the particles are in a liquid state and the temperature increases, then the state of matter will change, and it will become a gas. This will change the shape; the particles’ motion will increase, and the volume will increase.
In Step 6 molecules ‘break away’ from the surface of a liquid because they have gained energy and therefore their motion increases as they change from a liquid to a gas. This does not only happen at the boiling point of the liquid.
Exercise 1.1
- What are the three phases of matter?
- Identify the state of matter in each case:
- Particles which have lots of energy and a weak force of attraction between them.
- Particles that form 3-D lattice structures.
- Particles that can move past one other, take the shape of the bottom of a container, and have a medium density?
The full solutions are at the end of the unit.
The particle theory of matter
The particle theory of matter states that:
- All matter is made up of particles.
- Particles are in constant motion.
- Temperature affects the speed at which particles move.
- Particles have forces of attraction between them.
Activity 1.3: Investigate how heat affects the speed of movement of particles in a liquid
Time required: 10 minutes
What you will need:
- 2 transparent glasses or beakers
- food colouring
- hot and cold water
What to do:
- Half fill one glass or beaker with water from the cold tap and half fill the second glass or beaker with hot water. (Be careful and do not burn yourself!)
- Add a drop of food colouring to the water in both glasses or beakers at the same time and observe what happens for 5 minutes.
What did you find?
The particles in the hot water were moving fast and transferred some of their heat to the food colouring particles. All the particles were moving faster so the food colouring mixed faster. The particles in the cold water have less energy so the food colouring will spread out at a much slower rate.
Temperature is a measure of the average kinetic energy, and heat energy is transferred from the hotter particles to the cooler ones.
Temperature affects the speed at which particles move.
Water is made of one type of particle, and food colouring a different type of particle. When they are put together, they make a mixture.
All matter is made up of particles.
The particles in both the water and the food colouring are moving. When the food colouring is added, the particles in the food colouring move to spread out into the water. This is called .
Particles are in constant motion.
The food colouring can mix with the water because there are spaces between the water particles. The different particles are attracted to each other, therefore they mixed.
Particles have forces of attraction between them.
Exercise 1.2
- Give a definition for:
- Diffusion
- Temperature
- What is the particle theory of matter?
The full solutions are at the end of the unit.
Changing phase
You will now do a practical investigation.
Activity 1.4: Investigate the conditions under which particles of matter can change phase
Time required: 20 minutes
What you will need:
- a few ice cubes
- 2 transparent glasses or 2 transparent bottles with the tops cut off
- a small pot with a lid
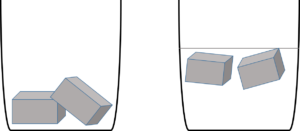
What to do:
- Put ice cubes into each glass. Half fill one of the glasses with some tap water.
- Do you notice that the ice floats? Do you know why?
- In which glass do you think the ice will melt faster? Can you explain why?
- Leave the glasses for about 15 minutes.
- Look at the glasses. What has changed?
- What do you notice about the state and volume of the of ice/water in each of the glasses?
- Pour the ice and the liquid from both glasses into a small pot. Heat the pot on a stove. Observe what happens in the pot. After a few minutes, the ice should have completely melted, and you should see steam coming into the air. Carefully hold the pot lid over the steam.
- What happens on the lid?
- Can you explain why?
What did you find?
-
- Ice floats because it is less dense than the water. This is because air bubbles get trapped in the ice as it freezes.
- The ice will melt faster in the warmer water, because there is more kinetic energy in the warm water. This gives kinetic energy to the particles in the ice more quickly, allowing them to have the energy to break the bonds holding them to the other particles in the ice, and change from a solid to a liquid.
- After about 15 minutes, the ice in both glasses should have begun to melt.
- The ice in the warmer water should have completely melted and changed from a solid to a liquid. The volume of liquid in the glass should have increased.
- As the liquid heats up, the motion of the particles will increase i.e. the particles will gain more kinetic energy. This allows them to break the bonds between the other liquid particles and change from a liquid to a gas. You are able to see this as the steam escapes.
You know that ice cream will melt on a hot day, and you have seen steam rising from a hot cup of tea on a cold day. This happens because substances can change phase from a solid to a liquid, a liquid to a gas, a solid to a gas, and vice versa.
All matter can change from one phase to another phase by adding or removing energy, usually in the form of heat energy. Adding heat energy increases the kinetic energy the particles of a substance have and removing heat energy reduces the kinetic energy the particles of a substance have.
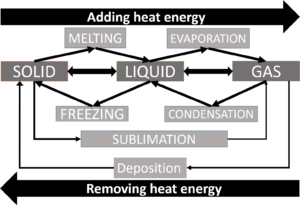
Adding energy
If you heat a solid, the particles stay the same, but they vibrate faster. As a result, they move slightly further apart, so the solid expands. If you carry on applying heat, the forces of attraction in the solid are weakened, and the solid melts and becomes a liquid. This also will change the volume and the shape of the substance.
This change happens at the solid’s .
If you carry on heating the liquid, the particles move around even more quickly. As the liquid gets hotter, the forces of attraction holding the particles together in the liquid break, and the particles are free. The liquid boils and becomes a gas. This is called and this change happens at the liquid’s . This also will change the volume and the shape of the substance.
Some substances can change from their solid phase straight to their gas phase without going through the liquid phase. This is called . Dry ice, which is frozen carbon dioxide, does this at room temperature, as does a substance called naphthalene.
Take note!
Liquids do not need to be at their boiling point before they will change into a gas. Particles at the surface can change to a gas if they have absorbed enough energy. Pure substances always melt and boil at certain fixed temperatures. The melting point of pure water is 0 ˚C, and the boiling point is 100 ˚C.
Removing energy
When a substance , it changes from a gas to a liquid. This requires energy to be removed. The temperature at which a liquid condenses is the same as its boiling point. The particles move less because they are losing kinetic energy. The volume and shape of the substance will also change as it becomes a liquid.
When a substance freezes, it changes from a liquid to a solid. This requires kinetic energy to be removed. A substance’s is the same temperature as its melting point. The particles move less because they are losing kinetic energy and the shape and volume of the substance will change as it becomes a solid.
is when a gaseous substance changes into a solid substance without a liquid phase. This is how frost is formed. Water vapour in the air changes into solid ice because lots of kinetic energy is removed as the air temperature drops in winter.
Summary of the phase changes
The table below summarises the phase changes of substances when energy is removed or added.
| Change of state | Energy added or removed | Temperature | Change of state |
| Solid to liquid | Added | Increases | Melting |
| Liquid to gas | Added | Increases | Evaporation |
| Solid to gas | Added | Increases | Sublimation |
| Gas to liquid | Removed | Decreases | Condensation |
| Liquid to solid | Removed | Decreases | Freezing |
| Gas to solid | Removed | Decreases | Deposition |
Exercise 1.3
- Ice cream will melt if it is not stored in a freezer. Explain why this happens.
- Wet washing will dry on a washing line, and rain puddles will dry out eventually. Why does this happen? Explain your answer.
The full solutions are at the end of the unit.
Summary
In this unit you have learnt the following:
- All matter is made of tiny particles that are constantly moving. This is the particle theory of matter.
- Matter exists as a solid, liquid, gas, or plasma.
- A solid has a fixed volume, fixed shape, and little energy.
- A liquid has a fixed volume, takes the shape of the bottom of the container it is in, and its particles have some energy.
- A gas takes the shape of the whole container it is in, has no fixed volume and the particles have lots of energy.
- Solids, liquids and gases can change state if energy is either removed or added to their particles.
- All matter is made of particles that are constantly moving.
- Particles move because they have energy.
- Particles’ energy increases with increasing temperature.
- Particles in the solid state have the least amount of energy compared to a liquid or gas.
- Particles in the liquid state have more energy than a solid, but less than a gas.
- Particles in the gaseous state have the most energy compared to a solid or liquid.
- The melting point of a solid is the temperature at which it will change to a liquid.
- The boiling point of a liquid is the temperature at which it will change to a gas.
Unit 1: Assessment
Suggested time to complete: 30 minutes
- Give one word or term for each of the following descriptions.
- The change in phase from a solid to a gas.
- The change in phase from liquid to gas.
- Water has a boiling point of 100 °C.
- Define the term ‘boiling point’.
- What change in phase takes place when a liquid reaches its boiling point?
- Describe a solid in terms of the kinetic particle theory.
- Refer to the table below which gives the melting and boiling points of a number of elements and then answer the questions that follow. (Data sourced from: www.chemicalelements.com)
| Element | Melting point °C | Boiling point °C |
| Copper | [latex]\scriptsize 1~083[/latex] | [latex]\scriptsize 2~567[/latex] |
| Magnesium | [latex]\scriptsize 650[/latex] | [latex]\scriptsize 1~107[/latex] |
| Oxygen | [latex]\scriptsize -218.4[/latex] | [latex]\scriptsize -183[/latex] |
| Carbon | [latex]\scriptsize 3~500[/latex] | [latex]\scriptsize 4~827[/latex] |
| Helium | [latex]\scriptsize -272[/latex] | [latex]\scriptsize -268.6[/latex] |
| Sulphur | [latex]\scriptsize 112.8[/latex] | [latex]\scriptsize 444.6[/latex] |
-
- What state of matter (i.e. solid, liquid or gas) will each of these elements be in at an average room temperature of 25 °C?
- Which of these elements has the strongest forces between its atoms? Give a reason for your answer.
- Which of these elements has the weakest forces between its atoms? Give a reason for your answer.
- In your notebook, draw particle diagrams to show what water will look like in the following phase:
- solid
- liquid
- gas
The full solutions are at the end of the unit.
Unit 1: Solutions
Exercise 1.1
- Solid, liquid and gas
- gas
- solid
- liquid
Exercise 1.2
-
- Diffusion – the movement of particles from an area of high concentration to an area of low concentration until they are equally spread.
- Temperature – a measure of how much kinetic energy a particle has.
- Particle theory of matter – everything is made of matter, which is constantly moving.
Exercise 1.3
- The particles in the ice cream will absorb thermal energy from the air surrounding it. This will give the energy needed for the particles to start moving away from each other and become a liquid.
- Liquid particles will evaporate when they have enough energy. The liquid particles in wet clothes and in puddles will change into gas particles when they have absorbed enough energy from their surroundings.
Unit 1: Assessment
-
- Sublimation
- Evaporation
- Boiling point is the temperature at which a liquid will change to a gas.
- Evaporation
- A solid has very little kinetic energy and its particles will only vibrate around a fixed position.
- .
Element Melting point °C Boiling point °C State at 25 °C Copper [latex]\scriptsize 1~083[/latex] [latex]\scriptsize 2~567[/latex] Solid Magnesium [latex]\scriptsize 650[/latex] [latex]\scriptsize 1~107[/latex] Solid Oxygen [latex]\scriptsize -218.4[/latex] [latex]\scriptsize -183[/latex] Gas Carbon [latex]\scriptsize 3~500[/latex] [latex]\scriptsize 4~827[/latex] Solid Helium [latex]\scriptsize -272[/latex] [latex]\scriptsize -268.6[/latex] Gas Sulphur [latex]\scriptsize 112.8[/latex] [latex]\scriptsize 444.6[/latex] Solid - Copper, because the difference between its melting point and boiling point is the highest, which means that more energy is needed to break the forces of attraction between each particle so that it can change state.
- Helium. The difference between the melting and boiling points is the lowest so less energy is needed to break apart the forces of attraction between the particles.
- .
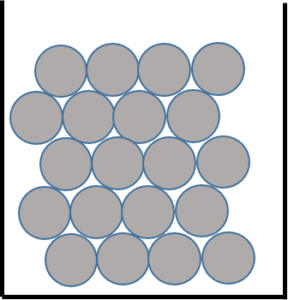
- .
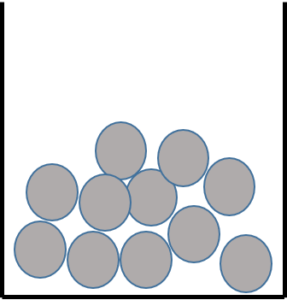
- .
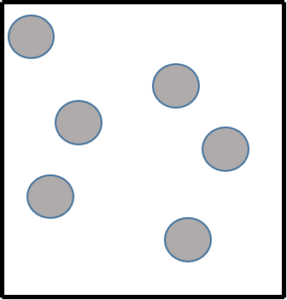
- .
Media Attributions
- Marbles © NASCA is licensed under a CC BY (Attribution) license
- Marbles 2 © NASCA is licensed under a CC BY (Attribution) license
- Marbles 3 © NASCA is licensed under a CC BY (Attribution) license
- Solid, Liquid Gas © Openstax is licensed under a CC BY (Attribution) license
- Lattice Structure © Prolineserver is licensed under a CC BY-SA (Attribution ShareAlike) license
- Plasma Torch © Hypertherm is licensed under a CC BY (Attribution) license
- Particles solid © DHET is licensed under a CC BY (Attribution) license
- Particles liquid © DHET is licensed under a CC BY (Attribution) license
- Particles gas © DHET is licensed under a CC BY (Attribution) license
- ice floating © DHET is licensed under a CC BY (Attribution) license
- Change of state flow diagram © DHET is licensed under a CC BY (Attribution) license
solid, liquid, gas
a measure of the kinetic energy that particles have
the energy an object has because of its motion
the movement of particles from an area of high concentration to an area of low concentration until they are equally spread
the temperature at which a solid will change to a liquid. This requires energy to be applied
change of phase from liquid to gas when heated
the temperature at which a liquid will change state to a gas. This requires energy to be put in and the particles will be able to move more
when substances change from solid phase straight to gas phase without going through the liquid phase
changes phase from gas to liquid when cooled
temperature at which a liquid will change to a to solid when cooled
when a gaseous substance changes into a solid substance without a liquid phase
