Matter and materials: Identify, describe, and classify matter according to different macroscopic properties
Unit 2: Properties of Materials
Emma Harrage
Unit outcomes
By the end of this unit you will be able to:
- Tell the difference between materials because of their macroscopic properties such as:
- thermal conductors and insulators
- electrical conductors and insulators
- metals and non-metals
- density
- acids and bases
- magnetic materials.
Introduction
In this unit you will learn about different materials by investigating and observing the behaviour of their properties. This will include learning about the differences between metals and non-metals; whether they are isolators or conductors of electricity and heat, whether they are magnetic, how dense they are and whether they are acidic or basic.
Characteristics of materials
Materials can be divided into three main groups according to their macroscopic properties. These groups are metals, non-metals, and metalloids.
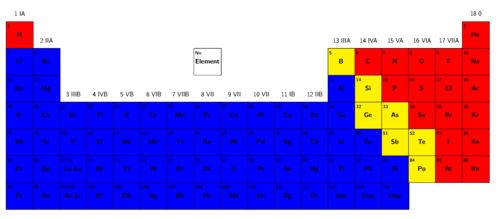
If you look at the periodic table in Figure 1, the metals can be found on the left-hand side of the table, shown in blue, and the metalloids and non-metals can be found on the right-hand side. The non-metals are indicated in red and the metalloids are shown in yellow. You should also notice that there are many more metals than non-metals.
Metals include copper, gold, silver, potassium, and lead. Non-metals include oxygen, carbon, helium and nitrogen, and metalloids include silicon and boron.
Properties of metals
These are the general macroscopic properties of metals:
- Metals are good conductors of heat and are therefore used to make cooking utensils such as pots and pans.
- Metals are good conductors of electricity and are therefore used in electrical conducting wires. Most wiring is made from copper because it is particularly good at conducting electricity.
- Metals have a characteristic shiny appearance and are often used to make jewellery.
- Metals can be ductile and malleable. Malleable means that they can be bent into shape without breaking and ductile means they can be stretched into thin wires.
- Metals usually have a high melting point and are used to make cooking pots and other equipment that needs to become extremely hot, without melting or catching fire.
- Almost all metals are solid at room temperature which means they have a high density. Mercury is the only metal that is a liquid at room temperature.
- Only three metals iron, cobalt and nickel are magnetic, the others are non-magnetic.
Properties of non-metals
Non-metals include helium, carbon, oxygen, and iodine.
These are the general macroscopic properties of non-metals:
- Non-metals are isolators of electricity which means they do not allow electricity to pass through them.
- Non-metals are heat isolators which means that they will not allow heat to travel through them easily.
- The density of a non-metal is dependent on its state of matter at room temperature. Most non-metals are either solid or gases at room temperature. Only bromine is a liquid.
- None of the non-metals are magnetic.
Properties of metalloids
Metalloids are all solid at room temperature and have mostly non-metallic properties. One of their distinguishing characteristics is that their conductivity increases as their temperature increases. This is the opposite of what happens in metals. This property is known as semi conductance and the materials are called semi-conductors. Semi-conductors are important in digital electronics, such as computers and cellphones. Metalloids include elements such as silicon and germanium.
Electrical Conductors and Isolators
An is a material that will allow an electrical current to pass through it and an is a material that will not allow an electrical current to travel through it.
Electrical conductors are usually metals. Copper is one of the best electrical conductors, and therefore it is used to make conducting wire. Silver has an even higher electrical conductivity than copper, but silver is too expensive to use for general electrical wiring. Graphite is the only non-metal that will conduct electricity.
Activity 2.1: Investigate the electrical conductivity of different materials
Time required: 30 minutes
What you will need:
- two or three batteries
- a light bulb
- crocodile clips
- wire leads
- a selection of test substances (e.g. piece of plastic, aluminium can, metal pencil sharpener, magnet, wood, chalk, cloth, a pencil sharpened at both ends)
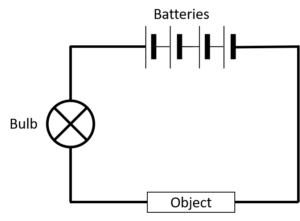
What to do:
- Set up the circuit as shown in the diagram.
- Place the test substances one by one between the crocodile clips and see what happens to the light bulb.
If the light bulb shines it means that a current is flowing and the substance you are testing is an electrical conductor.
Draw a table in your notebook to record your results, use the headings as shown in the table below:
| Test substance | Metal/non-metal | Light on/off | Conductor or isolator? |
| 0 |
Write a conclusion based on your results. When you write your conclusion, remember to include your results and explain why you got those results.
What did you find?
For the substances that were tested, the metals were able to conduct electricity and the non-metals, except graphite were not. Metals are good electrical conductors and non-metals are not.
| Test substance | Metal/non-metal | Light on/off | Conductor or isolator? |
| Paper clip | Metal | On | Conductor |
| Piece of plastic | Non-metal | Off | Isolator |
| Iron nail | Metal | On | Conductor |
| Pencil sharpened at both ends | Non-metal | On | Conductor |
| Fork | Metal | On | Conductor |
| Mug | Non-metal | Off | Isolator |
Thermal conductors and isolators
A is a material that allows energy in the form of heat to be transferred within the material, without any movement of the material itself.
A is a material that does not allow a transfer of heat energy. Materials that are poor thermal conductors can also be described as being good thermal isolators.
Take note!
A material with a high thermal conductivity value will lose heat more quickly because it is a good conductor.
Activity 2.2: Demonstrate the ability of different materials to conduct heat materials
Time required: 15 minutes
What you will need:
- 2 glasses or beakers
- 1 plastic spoon and 1 metal spoon
- hot water
- a timer
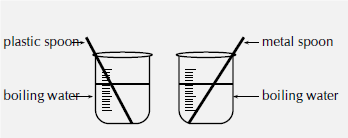
What to do:
- Set up the apparatus as shown in the diagram.
- CAREFULLY pour hot water into both glasses or beakers.
- Place a spoon in each glass and take note of which spoon is the hottest after 30 seconds.
What did you find?
The metal spoon is the hottest. This is because metals are thermal conductors. The plastic spoon is not very hot. This is because non-metals are thermal isolators.
Metals are generally good conductors of heat and non-metals are good isolators of heat. A material that is a good conductor of heat will lose heat more quickly.
Exercise 2.1
Use the information in the table to answer the following questions.
| Material | Thermal Conductivity [latex]\scriptsize (W.m^{-1}.K^{-1})[/latex] |
| Concrete | [latex]\scriptsize 0.9 - 2[/latex] |
| Air | [latex]\scriptsize 0.024[/latex] |
| Polystyrene | [latex]\scriptsize 0.03[/latex] |
| Wood | [latex]\scriptsize 0.04 - 0.12[/latex] |
| Glass | [latex]\scriptsize 1.05[/latex] |
| Red brick | [latex]\scriptsize 0.69[/latex] |
| Polyethylene (Plastic) | [latex]\scriptsize 0.42 - 0.51[/latex] |
| Stainless steel | [latex]\scriptsize 16[/latex] |
| Straw | [latex]\scriptsize 0.052[/latex] |
| Copper | [latex]\scriptsize 384.1[/latex] |
| Diamond | [latex]\scriptsize 895 - 1~300[/latex] |
| Water | [latex]\scriptsize 0.61[/latex] |
| Cast iron | [latex]\scriptsize 52[/latex] |
- Using the information in the table above, name two materials that are very good thermal conductors.
- Using the information in the table, name any two materials that are good isolators.
- Explain why:
- red brick is a better choice than concrete for building houses that need less internal heating
- stainless steel is good for making cooking pots
- From the information in the table, which metal would be better than stainless steel to use for cooking pots?
The full solutions are at the end of the unit.
Magnetism
Magnetism is a force that certain kinds of objects, which are called ‘magnetic’, can exert on each other without physically touching. A magnetic object is surrounded by a magnetic field that gets weaker as one moves further away from the object.
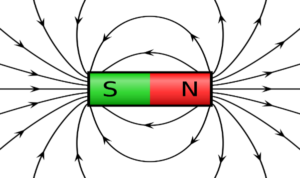
A metal is said to be ferromagnetic if it can be magnetised, which means made into a magnet.
Some metals keep their magnetism for longer than others. For example, iron loses its magnetism quite quickly if it is taken away from the magnet. Steel on the other hand will stay magnetic for a longer time. Steel is often used to make permanent magnets that can be used for a variety of purposes.
Magnets are used to sort metals in a scrap yard, in compasses to find direction, in the magnetic strips of ATM cards where information must be stored, in computers and TVs, as well as in generators and electric motors.
Relative density
is a measure of how much ‘stuff’ a substance has in a particular volume; it is an object’s mass per unit volume. An object’s density is measured relative to the density of water. Water has a density of 1g/cm3
Metals tend to be more dense than non-metals because they have more particles squashed into a given space. Gases have the lowest density.
Relative density is defined as the mass of a particular volume of a substance when compared with the mass of an equal volume of water at 4 °C.
| Material | Density (gram/cm3) |
| Water | [latex]\scriptsize 1.0[/latex] |
| Ethanol | [latex]\scriptsize 0.78[/latex] |
| Ice cube | [latex]\scriptsize 0.92[/latex] |
| Sunflower oil | [latex]\scriptsize 0.923[/latex] |
| Milk | [latex]\scriptsize 1.03[/latex] |
| Sodium chloride | [latex]\scriptsize 2.17[/latex] |
| Mercury | [latex]\scriptsize 13.5[/latex] |
| Aluminium | [latex]\scriptsize 2.7[/latex] |
| Copper | [latex]\scriptsize 8.9[/latex] |
| Cement | [latex]\scriptsize 3.1[/latex] |
| Gold | [latex]\scriptsize 19.3[/latex] |
Knowing the density of an object can allow you to work out if an object will float or sink in liquids. For example, gold will sink in water because it is denser, but an ice cube will float on water because it is less dense.
Exercise 2.2
- Using the information in the table above, list the substances in descending order of density.
- If you fill a glass with water, which of the following objects would sink or float in it?
- copper
- sunflower oil
- cement
The full solutions are at the end of the unit.
Acids and bases
A substance can be classified as an acid, a base, or a neutral substance according to the pH scale. We use acids and bases all the time; vinegar and lemon juice are weak acids, soap and shampoo are weak bases.
An is a substance that has a sour taste, although acids used in the laboratory should never be tasted. The strength of an acid varies from a weak acid, such as lemon juice, to a strong acid, such as sulphuric acid. Strong acids like sulphuric acid are used to clean cement and make fertilisers. Nitric acid is used to make explosives, and hydrochloric acid is used to make type of plastic called PVC.
A is the chemical opposite of an acid. Bases have a soapy feel and taste bitter. The strength of a base varies from being weak, for example sea water, to being very strong, for example drain cleaner. Sodium hydroxide is a strong base and is used to make soap and paper, and calcium hydroxide is used to clean sulphur dioxide fumes from industrial power plants.
Acids and bases will cause a particular colour change when a substance called universal indicator is added to them. The colour changes according to the strength of the substance.
As you can see in Figure 3, a strong acid turns universal indicator red, a weak acid turns the indicator yellow, a strong base turns the indicator purple and a weak base will turn the indicator blue. A substance will turn universal indicator green.
Acids can have a pH of 1 – 6 and bases can have a pH of 8 – 14. A neutral substance will have a pH of 7.
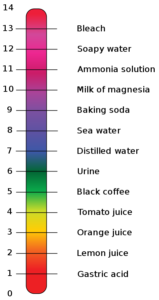
Summary
In this unit you have learnt the following:
- Metals are good electrical and thermal conductors, they have a shiny lustre, they are malleable and ductile, and they have a high melting point.
- Metals have a high density.
- The properties of metals make them useful for making electrical wires, cooking utensils, jewellery, and for many other applications.
- Matter can be classified into electrical conductors, semi-conductors, and isolators.
- An electrical conductor allows an electrical current to pass through it. Most metals are good electrical conductors.
- An electrical isolator is a non-conducting material that does not carry any charge. Examples are plastic, wood, cotton material and ceramic.
- Materials may also be classified as thermal conductors or thermal isolators depending on whether or not they are able to conduct heat.
- Materials may also be magnetic or non-magnetic. Magnetism is a force that certain kinds of objects, which are called ‘magnetic’ objects, can exert on each other without physically touching. A magnetic object is surrounded by a magnetic ‘field’ that gets weaker as one moves further away from the object.
- Materials have density, relative to the density of water. Gold is denser than water and ethanol is less dense. So, gold will sink in water and ethanol will float on water.
- Liquid substances can be classified as acidic, basic or neutral depending on their ability to donate or accept protons. Acids have a pH from 1 – 6, neutral substances have a pH of 7 and bases have a pH 8 – 14.
Unit 2: Assessment
Suggested time to complete: 15 minutes
- For each of the following materials, say which of its properties enable it to carry out its function.
- ceramic teacup
- iron burglar bars
- wool blanket
- metal jewellery
- red bricks for building
- copper wires
- plastic coating around wires.
- You are given a test tube that contains a mixture of iron filings and sulphur. You are asked to weigh the amount of iron in the sample.
- Suggest one method that you could use to separate the iron filings from the sulphur.
- What property of metals allows you to do this?
- Predict whether these objects would float or sink in water (density of water = kg/m3):
Oil: [latex]\scriptsize 913[/latex] kg/m3
Ice: [latex]\scriptsize 881[/latex] kg/m3
Pumice stone: [latex]\scriptsize 721[/latex] kg/m3
Sand: [latex]\scriptsize 1~602[/latex] kg/m3
Blood: [latex]\scriptsize 721[/latex] kg/m3
Chalk: [latex]\scriptsize 1~121[/latex] kg/m3
Wax: [latex]\scriptsize 320[/latex] kg/m3 - Pure water has a pH of 7, but rainwater has a pH of 4.5 and tap water has a pH of 6.5. Using this information, complete the table:
| Substance | pH | Approximate colour on the pH Scale, using universal indicator | Acid, base or neutral |
| Pure water | 7 | ||
| Rainwater | 4.5 | ||
| Tap water | 6.5 | ||
| Sea water | 8.1 |
The full solutions are at the end of the unit.
Unit 2: Solutions
Exercise 2.1
- copper and diamond
- Can be any two from: concrete, air, water, polystyrene, wood, glass, red brick, polyethylene (plastic), straw.
-
- it has a lower thermal conductivity than concrete
- it is a good thermal conductor
- cast iron, copper, or diamond
Exercise 2.2
- Ethanol
Ice cube
Sunflower oil
Water
Milk
Sodium Chloride
Aluminium
Cement
Copper
Mercury
Gold -
- sink
- float
- sink
Unit 2: Assessment
-
- ceramic is a good thermal isolator
- iron is strong
- wool is a good thermal isolator
- metal is shiny, malleable, and ductile
- good thermal isolator
- good electrical conductor
- good electrical isolator
-
- magnetism
- iron is magnetic and sulphur is not
- .
Float Sink wax sand oil chalk ice Pumice stone blood - .
Substance pH Approximate colour on the pH Scale, using universal indicator Acid, base or neutral Pure water 7 Green Neutral Rainwater 4.5 Yellow Acid Tap water 6.5 Blue Base Sea water 8.1 Blue Base
Media Attributions
- PT metals and non metals © Siyavula is licensed under a CC BY (Attribution) license
- series circuit © Siyavula is licensed under a CC BY (Attribution) license
- thermal conductor investigation © Siyavula is licensed under a CC BY (Attribution) license
- Magnetic field © GEEK3 is licensed under a CC BY (Attribution) license
- pH Scale © Edward Stevens is licensed under a CC BY (Attribution) license
material which will allow an electrical current to pass through it
material which will not allow an electrical current to travel through it
material that allows energy in the form of heat, to be transferred within the material, without any movement of the material itself.
material that does not allow a transfer of heat energy
measure of how much ‘stuff’ a substance has in a particular volume
a substance which has a pH of below 7
a substance which has a pH of above 7
a substance which is neither acidic nor basic and has a pH of 7
