Matter and materials: Identify, describe and apply properties of the periodic table
Unit 3: The behaviour of elements in specific groups
Emma Harrage
Unit outcomes
By the end of this unit you will be able to:
- Give the names of groups 1, 2, 7 and 0 and identify the transition metal group.
- Predict the activity of elements in their groups.
- Recognise where the metals and non-metals are on the periodic table.
What you should know:
Before you start this unit, make sure you can:
- Recognise the arrangement of atoms in the periodic table according to atomic number and Identify and describe group, period, and periodicity in Subject outcome 5.3, Unit 1: The periodic table.
Introduction
In this unit you will learn about groups 1, 2, 17 and 18 on the periodic table and the transition metals. Groups 1 and 2 are metals and their reactivity increases as you go down the group. For example, lithium is less reactive in water than potassium. The transition metals are all very stable and include relatively well-known elements like iron, gold, and silver. Group 17 and Group 18 are non-metals, and their reactivity decreases as you go down the group, so iodine is less reactive than chlorine.
Note
On some periodic tables, the groups are labelled from Group 1 to Group 8. This numbering does not include the transition metals. Numbering of the groups which includes the transition metals from Group 1 to Group 18 is used in these books.
As we learnt in SO 5.3 Unit 1, the arrangement of the periodic table and the properties of each element in it is based on the atomic number and the arrangement of the electrons orbiting the nucleus.
Specific groups on the periodic table
Many of the have been given names, for example Group 1 is called the alkali metals, Group 2 the alkali earth metals, Group 18 is called the noble gases and Group 17 is called the halogens. Elements are placed in a group because they share similar behaviours and bond with other elements in a similar configuration. Elements in the same group share similar properties. The properties of elements can be predicted based on their location in the group.
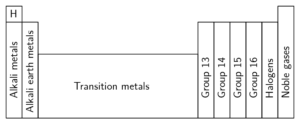
The alkali metals
If we look at Group 1, the alkali metals, the first three elements are lithium, sodium, and potassium. All three are soft, shiny metals and react with oxygen in the air. Lithium burns with a red flame, sodium with a yellow flame and potassium with a purple flame. These elements are stored in oil or paraffin to stop them from reacting with air. All three of these elements react vigorously with water.

Note
To see the reactions of alkali metals with water you can watch this video:
Scott Milmam: Alkali metals reacting with water. (Duration: 3.16)
The alkali earth metals
Group 2 elements, the alkali earth metals, include beryllium, magnesium, and calcium. All three will react with oxygen, but only calcium reacts readily with water. Magnesium reacts with steam but not water.

Note
The reactivity of elements in Group 1 and Group 2 increases as you go down the group. Caesium is the most reactive of all the metals and beryllium is the least reactive of the metals in Groups 1 or 2.
Note
For further explanation and to further understand some reactions of Group 2 you can watch this video:
Fuse Schools: What is Group 2? (Duration: 5.30)
The transition metals
The transition metals are found in the middle of the periodic table. Transition metals have several general properties. They are harder and less reactive than the alkali earth metals. They make colourful chemical compounds with other elements. Like other metals, they are electrical conductors. Some of the transition metals are necessary in trace amounts for good health, such as iron and zinc. Gold and silver are commonly used as jewellery because they are hard, malleable, and shiny.

Mercury is the only metal element that is a liquid at room temperature. It is heavy and dense, and it is highly toxic in large amounts. Mercury is most commonly used in fluorescent lamps and used to be used in thermometers and tooth fillings.

The halogens
The most reactive group of non-metals are the halogens in Group 17. Fluorine is the most reactive. It is a highly toxic, yellow gas at room temperature. It is the most electronegative element, so it is highly reactive.


Chlorine is a dense, toxic green gas at room temperature. Chlorine will bleach anything it comes into contact with. Compounds of chlorine are used in cleaning products and chemicals for swimming pools. Table salt, NaCl, consists of sodium and chlorine.
Bromine is a red-brown liquid at room temperature and is the only non-metal found in a liquid state at room temperature. It is highly reactive and toxic in large amounts.
Note
For further explanation and to further understand some reactions of Group 7, you can watch this video:
Fuse Schools: Group 7 – The halogens (Duration: 5.30).
The noble gases
Group 18 contains the noble gases. These are inert, stable elements because they have full outer shells. Helium is probably the most well-known noble gas because it is used in balloons. Helium is less dense than air, so this enables helium-filled balloons to float. If the gas is inhaled, then it changes your voice as you speak! Neon is used in electric signage and lighting.

Note
For further explanation on the different groups in the periodic table you can watch this video:
Fuse Schools: Periods and Groups in the periodic table (Duration: 2.53).
The activity of elements
To understand the activity of the various elements, we need to revise ionisation energy, atomic radius, and electronegativity.
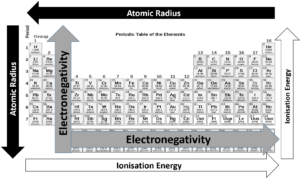
Going down the groups, the atomic radius increases, and the amount of energy needed to remove an outer electron decreases because the electrons are further from the nucleus and not held as tightly. This is important because how elements interact and react with each other depends on their ability to lose and gain electrons to make new compounds.
Moving from the left side of the periodic table to the right, the atomic radius decreases because each element has one additional proton and one additional electron. More protons means that electrons are pulled in more tightly towards the nucleus. This is the reason electronegativity increases from left to right.
The ionisation energy also increases from the left-hand side of the periodic table to the right. Metals tend to have a lower first ionisation energy level than non-metals, which means they will lose their valence electrons more readily.
Electronegativity decreases from the top of the column to the bottom. The melting point of the elements within a group also decreases from the top to the bottom of a group.
refers to how likely or vigorously an element is to react with other substances, and this is usually determined by the element’s ionisation energy and its electronegativity. Elements with high electronegativity will be very reactive, as will elements with low ionisation energy.
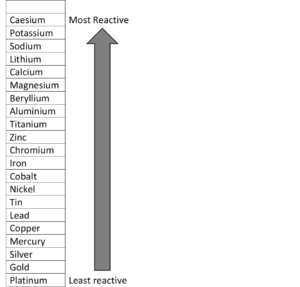
The reactivity of metals
As previously stated, the reactivity of metals in Group 1 and Group 2 increases down the group. The alkali metals are very reactive and there is a difference in the reactivity between sodium and caesium. Caesium has a lower ionisation energy and a lower electronegativity, so it is very reactive. Sodium has a greater ionisation energy than caesium, so it is not as reactive as caesium, but it is still very reactive.
If you consider the information in Table 1, you can see that the energy needed for caesium to lose its valence electron is lower than that of sodium. Therefore, caesium is more reactive than sodium. Its electronegativity is also lower because elements with a low ionisation energy will also have a lower electronegativity.
| Element | Group number | Period | Valence electrons | First ionisation energy KJ. Mol–1 |
Electronegativity |
| Caesium | [latex]\scriptsize 1[/latex] | [latex]\scriptsize 6[/latex] | [latex]\scriptsize 1[/latex] | [latex]\scriptsize 375.7[/latex] | [latex]\scriptsize 0.78[/latex] |
| Sodium | [latex]\scriptsize 1[/latex] | [latex]\scriptsize 3[/latex] | [latex]\scriptsize 1[/latex] | [latex]\scriptsize 495.8[/latex] | [latex]\scriptsize 0.93[/latex] |
| Lithium | [latex]\scriptsize 1[/latex] | [latex]\scriptsize 2[/latex] | [latex]\scriptsize 1[/latex] | [latex]\scriptsize 520.2[/latex] | [latex]\scriptsize 0.98[/latex] |
| Beryllium | [latex]\scriptsize 2[/latex] | [latex]\scriptsize 2[/latex] | [latex]\scriptsize 2[/latex] | [latex]\scriptsize 899.5[/latex] | [latex]\scriptsize 1.57[/latex] |
Table 1: The ionisation energy of certain metals
The reactivity of the more common metals are shown in Figure 10:
The reactivity of non-metals
Non-metals are more reactive as you travel up the group; oxygen is more reactive than sulfur and chlorine is more reactive than iodine. Non-metals generally have a higher electronegativity and first ionisation energy than metals.
| Element | Group number | Period | First ionisation energy KJ. Mol–1 |
Electronegativity |
| Hydrogen | – | [latex]\scriptsize 1[/latex] | [latex]\scriptsize 1312[/latex] | [latex]\scriptsize 2.20[/latex] |
| Fluorine | [latex]\scriptsize 17[/latex] | [latex]\scriptsize 2[/latex] | [latex]\scriptsize 1681[/latex] | [latex]\scriptsize 3.98[/latex] |
| Chlorine | [latex]\scriptsize 17[/latex] | [latex]\scriptsize 3[/latex] | [latex]\scriptsize 1251.2[/latex] | [latex]\scriptsize 3.16[/latex] |
| Bromine | [latex]\scriptsize 17[/latex] | [latex]\scriptsize 4[/latex] | [latex]\scriptsize 1139.9[/latex] | [latex]\scriptsize 2.96[/latex] |
| Helium | [latex]\scriptsize 18[/latex] | [latex]\scriptsize 1[/latex] | [latex]\scriptsize 2372.3[/latex] | – |
| Neon | [latex]\scriptsize 18[/latex] | [latex]\scriptsize 2[/latex] | [latex]\scriptsize 2080.7[/latex] | – |
| Argon | [latex]\scriptsize 18[/latex] | [latex]\scriptsize 3[/latex] | [latex]\scriptsize 1520.6[/latex] | – |
Table 2: The electronegativity of certain non-metals
You will see in Table 2 that there is no electronegativity listed for the three noble gases. This is because they are chemically inert.
Summary
In this unit you have learnt the following:
- The groups on the periodic table are labelled from 1 to 18. Group 1 is known as the alkali metals, Group 2 is known as the alkali earth metals, Group 17 is known as the halogens and Group 18 is known as the noble gases.
- The elements in a group have similar properties.
- Metal groups are more reactive as you move down the group, so potassium is more reactive than lithium, and calcium is more reactive than beryllium.
- Non-metals are less reactive as you go down the group. For the metals (Groups 1 to 13) the melting and boiling points increase as you go up the group. For the non-metals, the melting and boiling points decrease as you go up the group.
Unit 3: Assessment
Suggested time to complete: 20 minutes.
- Refer to the following list of elements:
Lithium (Li), Chlorine (Cl), Magnesium (Mg), Neon (Ne), Oxygen (O), Calcium (Ca), Carbon (C)
Which of the elements listed:- belongs to Group 1?
- is a halogen?
- is a noble gas?
- is an alkali metal?
- has an atomic number of 12?
- has all its energy orbitals full?
- will have chemical properties that are most similar?
- Are the following statements true or false? If they are false, correct the statement.
- The Group 1 elements are sometimes known as the alkali earth metals.
- The Group 18 elements are known as the noble gases.
- Group 7 elements are very unreactive.
- The transition elements are found between Groups 3 and 4.
- Lithium, sodium and potassium are the first three elements in Group 1. Explain why these three elements are in the same group.
- Using the information in the following table, list the elements in order of most reactive to least reactive:
Element Group number Period First ionisation energy
KJ. Mol–1Electronegativity Oxygen [latex]\scriptsize 16[/latex] [latex]\scriptsize 2[/latex] [latex]\scriptsize 1313.9[/latex] [latex]\scriptsize 3.44[/latex] Sulfur [latex]\scriptsize 16[/latex] [latex]\scriptsize 3[/latex] [latex]\scriptsize 999.6[/latex] [latex]\scriptsize 2.58[/latex] Fluorine [latex]\scriptsize 17[/latex] [latex]\scriptsize 2[/latex] [latex]\scriptsize 1681[/latex] [latex]\scriptsize 3.98[/latex] Chlorine [latex]\scriptsize 17[/latex] [latex]\scriptsize 3[/latex] [latex]\scriptsize 1251.2[/latex] [latex]\scriptsize 3.16[/latex] Bromine [latex]\scriptsize 17[/latex] [latex]\scriptsize 4[/latex] [latex]\scriptsize 1139.9[/latex] [latex]\scriptsize 2.96[/latex]
The full solutions are at the end of the unit.
Unit 3: Solutions
Unit 3: Assessment
- .
- Lithium
- Chlorine
- Neon
- Lithium
- Magnesium
- Neon
- Calcium and magnesium
- .
- False. Group 1 are called the alkali metals.
- True
- False. Group 7 are very reactive elements.
- False. The transition elements are found between Groups 2 and 13.
- They have similar chemical properties and react in similar ways with water and oxygen.
- .
Fluorine
Chlorine
Oxygen
Bromine
Iodine
Sulfur
Media Attributions
- Groups Names PT © Depratment of Higher Education and Training is licensed under a CC BY (Attribution) license
- Lithium metal © Depratment of Higher Education and Training is licensed under a CC BY (Attribution) license
- Calcium metal © Department of Higher Education is licensed under a CC BY (Attribution) license
- Gold © GOKLuLe - DIY is licensed under a CC BY-SA (Attribution ShareAlike) license
- Mercury © Tomihahndorf is licensed under a CC BY-SA (Attribution ShareAlike) license
- Fluorine © Agnico-Eagle is licensed under a CC0 (Creative Commons Zero) license
- Chlorine © Chitrapa is licensed under a CC BY-SA (Attribution ShareAlike) license
- Neon lights © A is licensed under a CC BY-SA (Attribution ShareAlike) license
- Periodic table arrowed © Depratment of Higher Education and Training is licensed under a CC BY (Attribution) license
- Reactivity series © Prof B. G. Mueller is licensed under a CC BY-SA (Attribution ShareAlike) license
rows of elements arranged together because they have similar chemical properties
how vigorously an element will react with another substance.
