Matter and materials: Identify and describe particles
Unit 3: Bonding
Emma Harrage
Unit outcomes
By the end of this unit you will be able to:
- Identify, define and give examples of:
- a covalent bond
- an ionic bond
- a metallic bond.
- Explain the macroscopic and microscopic properties of bonding.
- Name and write chemical formulae for common substances like:
- water
- sodium chloride
- carbon dioxide
- sulphuric acid.
What you should know:
Before you start this unit, make sure you can:
- Confidently complete all the work in Subject outcome 5.2.
- Confidently complete all the work in Subject outcome 5.3.
- Confidently complete all the work in Subject outcome 5.4 Unit 1 and Unit 2.
Introduction
In this unit you will learn to identify and describe bonding between elements to form compounds. Bonds formed between metals and non-metals are ionic bonds. Bonds formed between two or more non-metals are covalent bonds. Bonds formed between metals are metallic bonds. You will also learn to write the chemical formulae of commonly used substances like water, carbon dioxide and acids such as sulphuric acid.
Chemical bonding
Atoms seldom exist on their own. More often, the things around us are made up of different atoms that have joined together. They join through . Chemical bonding is one of the most important processes in chemistry because it allows all sorts of different molecules and combinations of atoms to form, which then make up the complex objects in the world around us.
Atoms are more reactive, and therefore more likely to bond, when their outer electron orbitals are not full. Atoms are less reactive when these outer orbitals contain the maximum number of electrons, in other words when they have a stable electron configuration. This explains why the noble gases do not react.
A chemical bond is formed when atoms are held together by attractive forces. This attraction occurs when electrons are either shared between atoms, or when electrons are exchanged between the atoms that are involved in the bond. The sharing or exchange of electrons takes place so that the outer energy levels of the atoms involved are filled, making the atoms more stable. If an electron is shared, it means that it will spend its time moving in the electron orbitals around both atoms. If an electron is exchanged, it means that it is transferred from one atom to another. In other words, one atom gains an electron while the other atom loses an electron.
A chemical bond is the physical process that causes atoms and molecules to be attracted to each other and held together in more stable chemical compounds.
The type of bond that is formed depends on the elements that are involved:
- non-metals will bond with each other forming covalent bonds.
- non-metals and metals will bond forming .
- metals will bond with metals forming .
Covalent bonding
occurs between the atoms of non-metals. The outermost energy levels of the atoms overlap so that unpaired electrons in each of the bonding atoms can be shared.
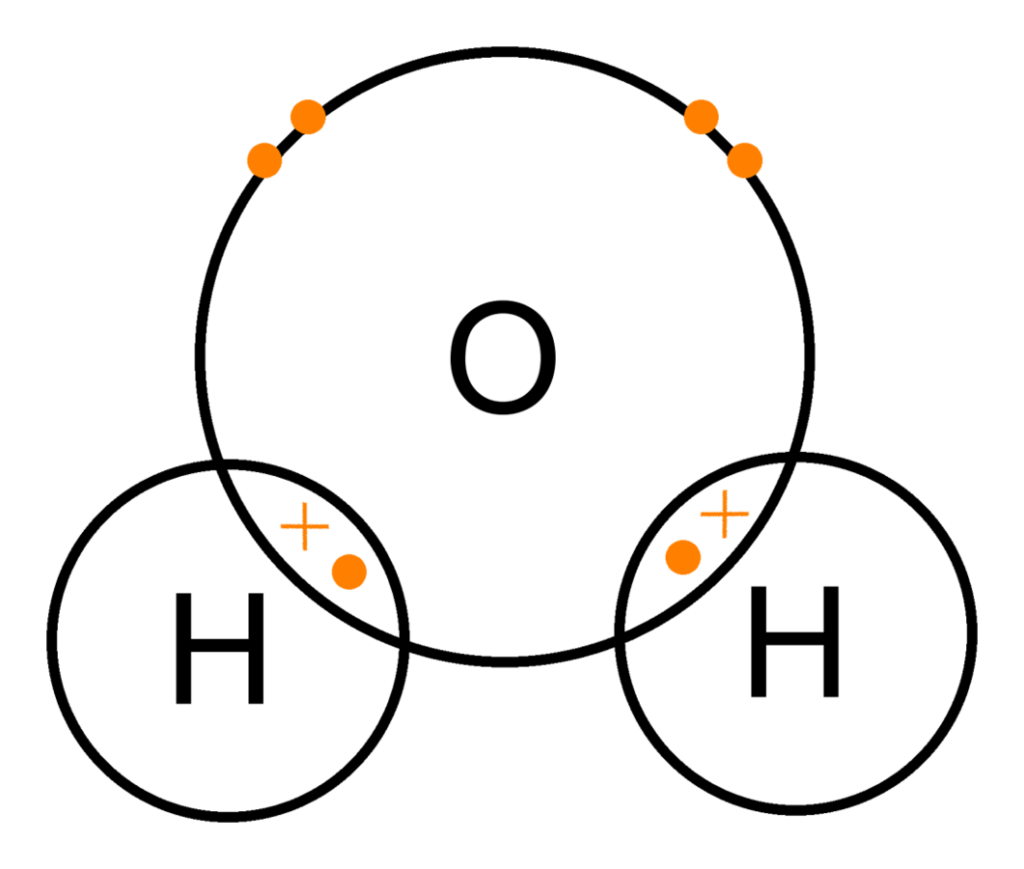
By overlapping orbitals, the outer energy shells of all the bonding atoms are filled. The shared electrons move in the orbitals around both atoms. As they move, there is an attraction between the negatively charged electrons and the positively charged nuclei. This attractive force holds the atoms together in a covalent bond.
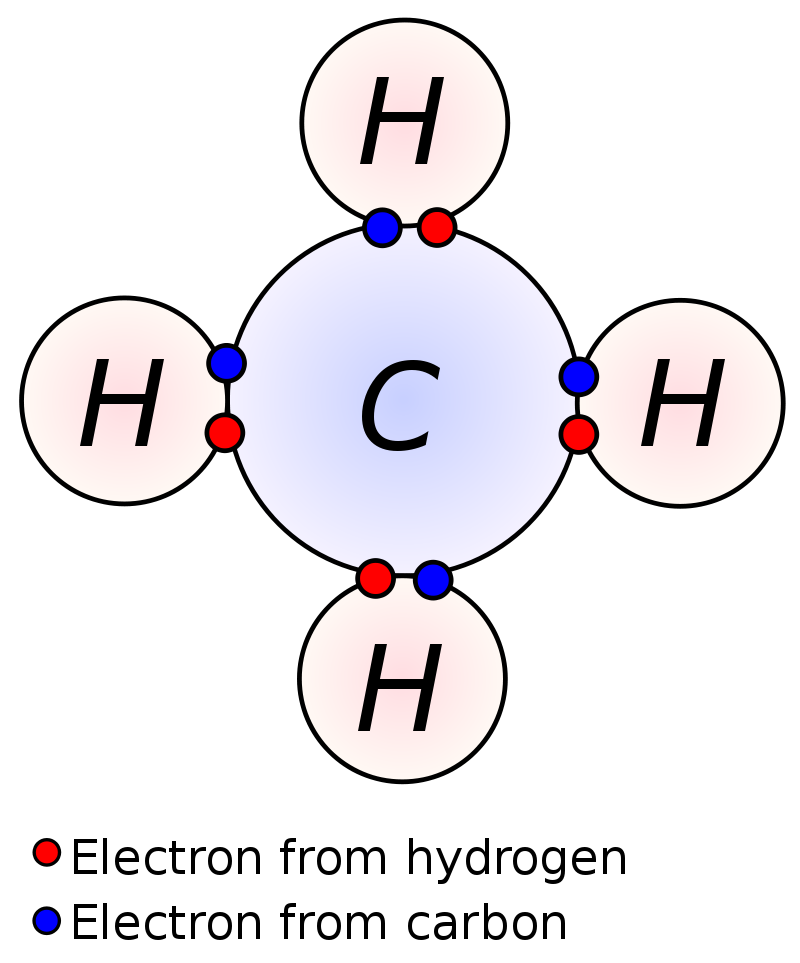
Example 1
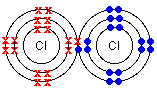
- Draw a shell diagram to explain the covalent bond in chlorine.
- Write out the electron configuration for chlorine.
- Work out how many valence electrons each chlorine atom has.
- Work out how many electrons each atom needs to have a stable electron configuration.
Solutions:
- Chlorine is diatomic. An atom will form a covalently bonded molecule with another atom, so it becomes more stable.
To draw a diagram like this:
-
- Draw the outer energy levels, fill in the electrons. (It is not necessary to draw all of the energy levels, you can just draw the outer energy level with the valence electrons.)
- Use dots to represent the electrons from one type of atom and use crosses for the other type of atom.
- Draw the outer most energy levels close together and place the shared electrons where the circles meet.
- Chlorine’s atomic number is [latex]\scriptsize 17[/latex] so its electron configuration will be [latex]\scriptsize 2,8,7[/latex].
- Each atom has [latex]\scriptsize 7[/latex] valence electrons. Remember, these are the electrons on the outermost energy level, and are the only electrons involved in bonding.
- Each chlorine atom needs [latex]\scriptsize 1[/latex] electron to have a full outer most energy level. So, the atoms will ‘share’ [latex]\scriptsize 1[/latex] electron. Remember, to be stable, each atom needs either [latex]\scriptsize 2[/latex] or [latex]\scriptsize 8[/latex] electrons on its outer most energy level.
Other examples of covalently bonded compounds can be seen in the table below:
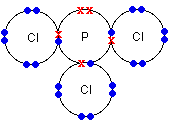 |
Phosphorus chloride (PCl3) |
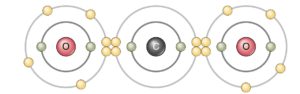 |
Carbon dioxide (CO2) |
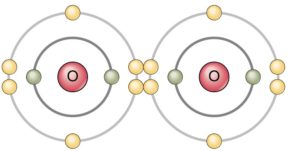 |
Oxygen (O2) |
Properties of covalent bonding
Covalent compounds have several properties that distinguish them from ionic compounds and metals.
- The melting and boiling points of covalent compounds are generally lower than those of ionic compounds.
- Covalent compounds are generally more flexible than ionic compounds. The molecules in covalent compounds can move around to some extent and can sometimes slide over each other.
- Covalent compounds are generally not very soluble in water, for example plastics are covalent compounds and many plastics are water resistant.
- Covalent compounds generally do not conduct electricity when dissolved in water, for example iodine dissolved in pure water does not conduct electricity.
Note
If you need further explanation of covalent bonding you can watch this video:
Fuse Schools: Covalent bonding of hydrogen, oxygen, and nitrogen (Duration: 3.24).
Exercise 3.1
In your notebooks, draw shell diagrams to show the bonding in:
- Hydrogen (H2)
- Hydrogen chloride (HCl)
- Ammonia (NH3)
The full solutions are at the end of the unit.
Ionic bonding
In ionic bonding electrons are transferred from one atom to another, unlike in covalent bonds where electrons are shared to achieve a stable electron configuration.
Ionic bonding is the complete transfer of valence electron(s) between atoms and is a type of chemical bond that generates two oppositely charged ions. Metals will donate electrons to non-metals and become positively charge ions and non-metals tend to readily accept electrons to achieve a stable configuration.
Bonding in Sodium fluoride (NaF)
Sodium and fluorine form an ionic bond to become sodium fluoride. Watch the animation to see how.
Sodium has the electron configuration [latex]\scriptsize 2,8,1[/latex]. To achieve a stable electron configuration, it needs to ‘lose’ 1 electron to have the configuration [latex]\scriptsize 2,8[/latex].
Fluorine has the electron configuration [latex]\scriptsize 2,7[/latex]. To achieve a stable configuration it needs to ‘gain’ 1 electron.
So, sodium will give its electron to fluorine and become a positively charge ion Na+ and fluorine will become negatively charged F–.
Bonding in Sodium chloride (NaCl)
Sodium [latex]\scriptsize 2,8,1[/latex] has 1 electron more than a stable structure [latex]\scriptsize 2,8[/latex]. If it gives away that electron it will become more stable.
Chlorine [latex]\scriptsize 2,8,7[/latex] has 1 electron short of a stable structure [latex]\scriptsize 2,8,8[/latex]. If it gains an electron from somewhere it too will become more stable. So, if a sodium atom gives an electron to a chlorine atom, both become more stable.
[latex]\scriptsize \begin{align} \text{Na }2,8,1 &\to \text{N}{{\text{a}}^{+}}2,8 \\ \text{Cl 2,8,7 } &\to \text{ C}{{\text{l}}^{-}}2,8,8 \\ \end{align}[/latex]
You need one sodium atom to provide the extra electron for one chlorine atom, so they combine 1:1. The formula is therefore NaCl.
The sodium atom has lost an electron, so it no longer has equal numbers of electrons and protons. Because it has one more proton than electron, it has a charge of +1. If an atom loses electrons, positive ions are formed. Positive ions are also called .
The chlorine atom has gained an electron, so it now has one more electron than protons. It therefore has a charge of –1. If an atom gains electrons, negative ions are formed. A negative ion is also called an .
In Sodium Chloride (NaCl), the sodium ions and chloride ions are held together by the strong electrostatic attractions between the positive and negative charges. Ionic substances are a combination of lots of ions bonded together into a giant molecule. The arrangement of ions in a regular, geometric structure is called a . So in fact, NaCl does not contain one Na and one Cl ion, but rather many of these two ions arranged in a crystal lattice where the ratio of Na to Cl ions is 1:1. The structure of the crystal lattice is shown in Figure 4.
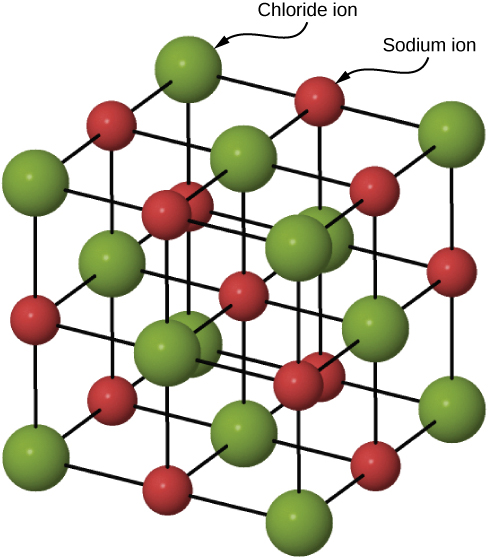
Bonding in Magnesium Oxide (MgO)
In Magnesium Oxide, the two elements magnesium and oxygen, lose and gain 2 electrons respectively in order to achieve a stable configuration.
[latex]\scriptsize \begin{align} \text{Mg 2,8,2 } &\to \text{ M}{{\text{g}}^{2+}}2,8 \\ \text{O 2,6 } &\to \text{ }{{\text{O}}^{2-}}2,8 \\ \end{align}[/latex]
The ionic bonding is therefore stronger than in sodium chloride because here you have an ion with a [latex]\scriptsize {}^{+}2[/latex] charge attracting an ion with a [latex]\scriptsize ~{}^{-}2[/latex] charge. The greater the charge, the greater the attraction. The formula of magnesium oxide is MgO.
Properties of ionic compounds
Ionic compounds have several distinguishing properties.
- Ions are arranged in a lattice structure.
- Ionic solids are crystalline at room temperature.
- The ionic bond is a strong electrostatic attraction. This means that ionic compounds are often hard and have high melting and boiling points.
- Ionic compounds are brittle, and bonds are broken along planes when the compound is put under pressure (stressed).
- Solid crystals do not conduct electricity, but ionic solutions do.
Note
If you need further explanation of ionic bonding you can watch this video:
Fuse Schools: What are ionic bonds? (Duration 2:54)
Exercise 3.2
For the following questions, choose the most correct answer:
- Ionic bonds form because:
- two ions of the same charge are attracted to each other.
- two ions of different charges are attracted to each other.
- two atoms share their electrons.
- Which of these is a property of an ionic compound?
- They have a low melting point.
- They are poor conductors of electricity.
- They form lattice structures.
- The symbol Li+ means?
- That this lithium isotope has 1 positive charge.
- That this lithium ion has 1 positive charge.
- That lithium has 1 valance electron.
- How many chloride ions are needed to cancel the [latex]\scriptsize {}^{+}2[/latex] charge of magnesium in magnesium chloride?
- 1
- 2
- 3
The full solutions are at the end of the unit.
Metallic bonding
The structure of a metallic bond is quite different to both covalent and ionic bonds. In a metallic bond, the valence electrons are delocalised, meaning that an atom’s electrons do not stay around that atom’s nucleus. In a metallic bond, the positive atomic nuclei are surrounded by a sea of delocalised electrons which are attracted to the various nuclei.
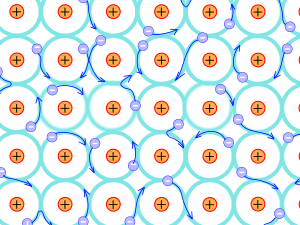
Writing chemical formulae
A is a short-hand way of writing out the names of compounds and elements. It is quicker and easier to write HCl than to write out hydrogen chloride. Using chemical formulae also shows how many atoms of a particular element are in different compounds.
The following are some guidelines for naming compounds:
- The compound name will always include the names of the elements that are part of it.
-
- A compound of iron (Fe) and sulfur (S) is iron sulfide (FeS).
- A compound of potassium (K) and bromine (Br) is potassium bromide (KBr).
- A compound of sodium (Na) and chlorine (Cl) is sodium chloride (NaCl).
- In a compound, the element that is on the left of the periodic table is used first when naming the compound. In the example of NaCl, sodium is a group 1 element on the left-hand side of the table, while chlorine is in group 17 on the right side of the table. Sodium therefore comes first in the compound name. The same is true for FeS and KBr.
- The symbols of the elements can be used to represent compounds. These are called chemical formulae, for example:
-
- FeS – iron sulfide
- NaCl – sodium chloride
- H2O – water.
- When numbers are written as subscripts in compounds (they are written below and to the right of the element symbol), this tells us how many atoms of that element there are in relation to other elements in the compound, for example:
-
- NO2 (nitrogen dioxide) – there are two oxygen atoms for every nitrogen atom.
- H2O (water) – there are two hydrogen atoms for every oxygen atom.
- CO (carbon monoxide) – there is one oxygen atom for every carbon atom.
- NO2 (nitrogen dioxide) – there are two oxygen atoms for every nitrogen atom.
- SO3 (sulfur trioxide) – there are three oxygen atoms for every sulfur atom.
- H2SO4 (sulfuric acid) – there are two hydrogen atoms for every one sulfur atom and for every four oxygen atoms.
- CO2 (carbon dioxide) – there are two oxygen atoms for every carbon atom.
Example 2
Write the chemical formula for sodium fluoride.
Solution
- List the ions involved: You will need to work out the electron configuration of both sodium and fluorine. From there you should know that sodium needs to lose 1 electron to become stable and fluorine needs to gain 1 electron to become stable. This means that the ions Na+ and F− will form.
- Write down the charges on the ions: The sodium ion has a charge of +1 and the fluoride ion has a charge of −1.
- Find the right combination: For every plus, we must have a minus. So, the +1 from sodium cancels out the −1 from fluoride. They combine in a 1:1 ratio.
- Therefore the chemical formula for sodium fluoride is NaF.
Example 3
Write the chemical formula for magnesium chloride.
Solution
- List the ions involved by working out the electron configuration for magnesium and chlorine, then working out how many electrons they each need to lose or gain to have a stable configuration: Mg2+ and Cl−.
- Find the right combination: Magnesium has a charge of +2 and would need two chlorides to balance the charge. They will combine in a 1:2 ratio. There is an easy way to find this ratio:
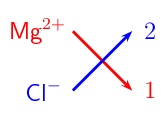
- Draw a cross as above, and then you can see that Mg2+ →1 and Cl− →2.
- Write down the formula: MgCl2.
Exercise 3.3
- Write the chemical formula for:
- calcium oxide
- potassium oxide
- Explain why carbon tetrafluoride has the chemical formula of CF4. You may draw a diagram to help with your explanation.
The full solutions are at the end of the unit.
Summary
In this unit you have learnt the following:
- A chemical bond is the physical process that causes atoms and molecules to be attracted to each other and held together in more stable chemical compounds.
- Atoms are more reactive, and therefore more likely to bond, when their outer electron orbitals are not full.
- Atoms are less reactive when these outer orbitals contain the maximum number of electrons.
- When atoms bond, electrons are either shared or exchanged.
- Covalent bonding occurs between the atoms of non-metals and involves a sharing of electrons so that the orbitals of the outermost energy levels in the atoms are filled.
- Properties of covalent compounds:
- low melting and boiling points
- insoluble in water
- softer and flexible
- insulators of electricity and heat.
- An ionic bond occurs between atoms where there is an exchange of electrons. The atoms are held together by the electrostatic force of attraction between the resulting oppositely charged ions.
- Ionic solids are arranged in a crystal lattice structure.
- Properties of ionic compounds:
- high melting and boiling points
- soluble in water
- harder and inflexible
- good conductors of electricity and heat.
- A metallic bond is the electrostatic attraction between the positively charged nuclei of metal atoms and the delocalised electrons in the metal.
- When naming compounds and writing their chemical formula, it is important to know the elements that are in the compound, how many atoms of each of these elements will combine in the compound and where the elements are in the periodic table.
Unit 3: Assessment
Suggested time to complete: 30 minutes
- Name the type of bonding in the following compounds or molecules:
-
- nitrogen (N2)
- lithium oxide (Li2O)
- vinegar/acetic acid (C2H4O2)
For questions 2 to 7, choose the most correct answer.
-
- A chemical bond in which one atom loses an electron to form a positive ion and the other atom gains an electron to form a negative ion is a/an:
- cation
- ionic bond
- isotope
- covalent bond
- What is a covalent bond?
- The transfer of electrons from one atom to another atom.
- The sharing of electrons equally between atoms of non-metals to attain a stable electronic configuration.
- Why do non-metals covalently bond?
- They want to create new chemical compounds.
- They want full electron shells.
- Because it is nature.
- What type of atoms (or ions) will form an ionic substance?
- two non-metals
- two metals
- a metal ion and a non-metal ion
- What type of ions do metals form?
- Metals form positive cations, for example Na1+.
- Metals form positive anions, for example: Na1+.
- Metals form negative anions, for example: Na1-.
- Ionic bonds form because:
- two ions of the same charge are attracted to each other.
- two ions of different charges are attracted to each other.
- two atoms share their electrons.
- two or more atoms share protons.
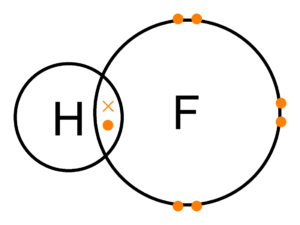
- Draw a shell diagram to show the covalent bonding in hydrogen fluoride.
- Explain why lithium oxide has the chemical formula Li2O.
- Copy the following table into your notebook and complete it:
Name of compound Name and number of elements in the compound Calcium oxide (CaO) Element:
Number:
Element:
Number:Potassium permanganate (KMnO4) Element:
Number:
Element:
Number:
Element:
Number:Nitric acid (HNO3) Element:
Number:
Element:
Number:
Element:
Number:
The full solutions are at the end of the unit.
Unit 3: Solutions
Exercise 3.1
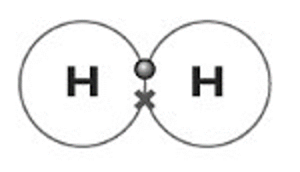
- Hydrogen. Hydrogen has 1 electron. In order to become stable, it will bond with another atom of hydrogen. The atoms will share the two electrons.
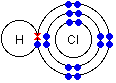
- Hydrogen chloride. Hydrogen has 1 electron. Chlorine has 7 electrons on its outermost energy level and needs 1 more electron to have a stable electron configuration. It will form a covalent bond with hydrogen by sharing 1 electron and the 1 electron from hydrogen.
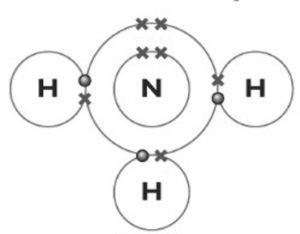
- Ammonia. Nitrogen needs to gain 3 electrons to have a stable configuration so it will bond with 3 hydrogen atoms, which have 1 electron each.
Exercise 3.2
- b – ionic bonds form between a negatively charged non-metal ion and a positively charged metal ion – opposites attract!
- c – the ions form a geometric lattice.
- b – lithium will lose 1 electron and become a positively charged ion.
- b – each chlorine ion needs to gain 1 electron for a stable configuration, so 2 chlorine ions will be needed to cancel out the [latex]\scriptsize {}^{+}2[/latex] charge.
- .
- calcium oxide will be CaO. Calcium forms a Ca2+ ion. Oxygen forms a O2–, so the ratio of atoms will be 1:1 and the formula will be CaO.
- potassium oxide will be K2O. Potassium forms a K+ ion because it will readily lose an electron. Oxygen forms a O2- ion so 2 K+ ions are needed to cancel out the −2 charge of the oxygen.
- Carbon tetrafluoride is CF4 because carbon needs 4 electrons for a stable electron configuration and fluorine needs 1 electron for a stable configuration. To fill up the outermost energy level of carbon you will need 4 fluorine atoms:
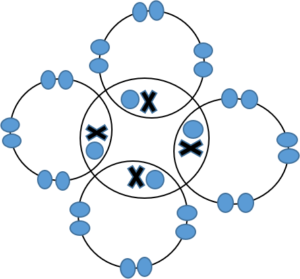
- .
- covalent
- ionic
- covalent
- b
- b
- b
- c
- a
- b
- Shell diagram to show the covalent bonding in hydrogen fluoride:
- Lithium has 1 valence electron, so will need to lose 1 electron to form a stable electron configuration and will form a Li+ ion. Oxygen has 6 valence electrons and needs to gain 2 electrons from lithium to form a stable electron configuration. Because oxygen needs 2 electrons, 2 lithium’s are needed, and oxygen will form a O2– ion.
- .
Name of compound Name and number of elements in the compound Calcium oxide (CaO) Element: calcium
Number: 1
Element: oxygen
Number: 1Potassium permanganate (KMnO4) Element: potassium
Number: 1
Element: manganese
Number: 1
Element: oxygen
Number: 1Nitric acid (HNO3) Element: hydrogen
Number: 1
Element: nitrogen
Number: 1
Element: oxygen
Number: 3
Media Attributions
- Water covalent bonding © Benjah-bmm27. is licensed under a CC0 (Creative Commons Zero) license
- Shell diagram of Methane © Dyna blast is licensed under a CC BY-SA (Attribution ShareAlike) license
- Chlorine simple covalent © Libre texts is licensed under a CC BY-NC-SA (Attribution NonCommercial ShareAlike) license
- Phosphorus chloride simple covalent © Libre texts is licensed under a CC BY-NC-SA (Attribution NonCommercial ShareAlike) license
- Simple covalent Carbon Dioxide © Rice University is licensed under a CC BY (Attribution) license
- simple covalent oxygen © Rice University is licensed under a CC BY (Attribution) license
- Sodium Chloride crystal © Benjah-bmm27 is licensed under a CC0 (Creative Commons Zero) license
- metallic_bonding © すじにくシチュー is licensed under a CC0 (Creative Commons Zero) license
- Chemical formula magnesium chloride © Siyavula is licensed under a CC BY (Attribution) license
- Shell diagram hydrogen.jpg © Benjah-bmm27 is licensed under a CC0 (Creative Commons Zero) license
- Hydrogen chloride simple covalent © Department of Higher Education and Training is licensed under a CC BY (Attribution) license
- Shell diagram ammonia © Department of Higher Education and Training is licensed under a CC BY (Attribution) license
- Carbon tetrachloride © Department of Higher Education and Training is licensed under a CC BY (Attribution) license
- Hydrogen-fluoride-2D-dot-cross © Benjah-bmm27 is licensed under a CC0 (Creative Commons Zero) license
bonding formed when different atoms join together
type of chemical bonds where electrons are lost or gained between atoms
type of chemical bonds between positively charged atoms and free electrons in a ‘soup’
type of chemical bonding in which pairs of electrons are shared between atoms
positively charged ions
negatively charged ions
geometric lattice formed by bonding ions
notation that shows the number of atoms and the names of atoms in a compound

