Magnetism and electricity: Identify, describe, and apply principles of electrostatics (static electricity)
Unit 1: What is charge?
Leigh Kleynhans
Unit 1 outcomes
By the end of this unit you will be able to:
- Identify two kinds of charge.
- Describe how an object becomes charged.
- Define the law of conservation of charge.
What you should know
Before you start this unit, make sure you can:
- Identify and apply the principles of forces, as covered in Subject outcome 2.2, Unit 1.
Introduction
In this unit[1] you will learn what ‘charge’ is and what it means when we say an object is or becomes charged.
Electrostatics is the study of electric charge which is at rest or static (not moving). In this unit we will look at some of the basic principles of electrostatics as well as the principle of conservation of charge.
What is charge?
All objects surrounding us (including people!) contain large amounts of electric charge. There are two types of electric charge: positive charge and negative charge. If the same amounts of negative and positive charge are found in an object, there is no net charge, and the object is electrically neutral. If there is more of one type of charge than the other on the object, then the object is said to be electrically charged. Figure 1 below shows what the distribution of charges might look like for a neutral, positively charged and negatively charged object.

Positive charge is carried by the protons in material and negative charge by electrons. The overall charge of an object is usually due to changes in the number of electrons.
To make an object:
- positively charged, electrons are removed making the object electron .
- negatively charged, electrons are added giving the object an of electrons.
So in practise what happens is that the number of positive charges (protons) remains the same and the number of negative charges (electrons) changes:
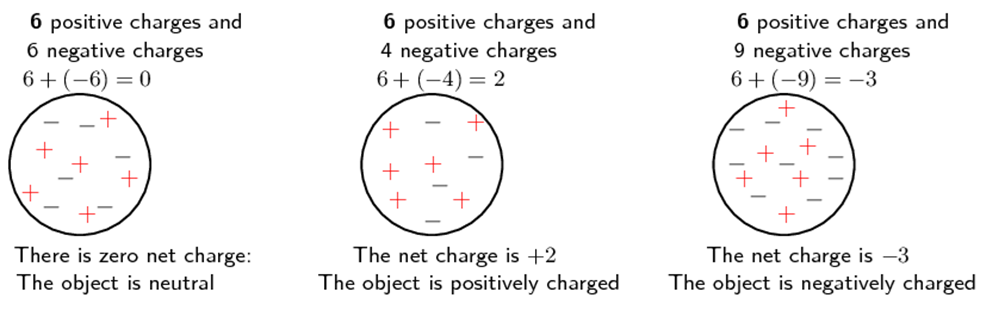
Note
We represent the positive charge with a + and the negative charge with a −. These symbols illustrate the balance and changes that occur, not the actual number or location of the positive and negative charges. The charges are spread throughout the material and the real change happens by increasing or decreasing electrons on the surface of the materials.
How does an object become charged?
Objects may become charged in many ways, including coming into contact with or being rubbed by other objects. This means that they can gain or lose negative charge. For example, charging happens when you rub your feet against the carpet. When you rub your feet against the carpet, negative charge is transferred to you from the carpet. The carpet will then become positively charged by the same amount.
Another example is to take two neutral objects such as a plastic ruler and a cotton cloth. To begin with, the two objects are neutral (i.e. have the same amounts of positive and negative charge).
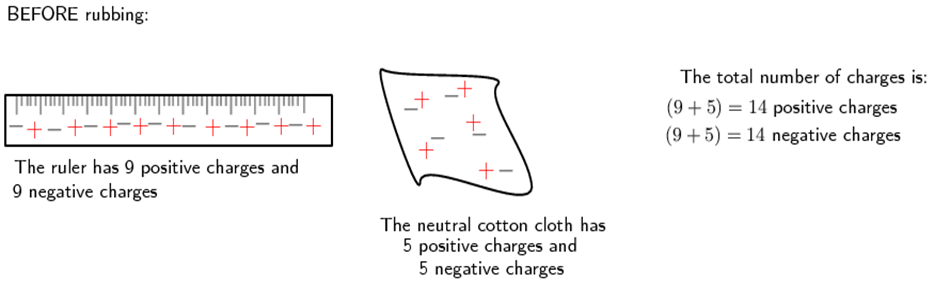
Now, if the cotton cloth is used to rub the ruler, negative charge is transferred from the cloth to the ruler. The ruler is now negatively charged (i.e. has an excess of electrons) and the cloth is positively charged (i.e. is electron deficient). If you count up all the positive and negative charges at the beginning and the end, there are still the same amount, i.e. total charge has been conserved!
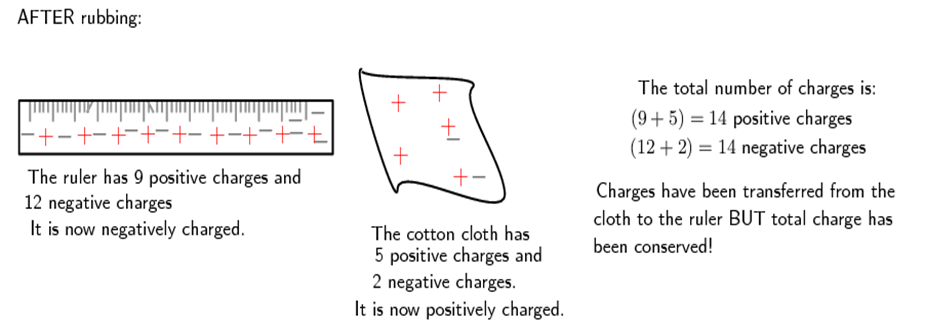
What determines the direction in which the negative charges move?
You now know that two objects made of different materials can be electrically charged by rubbing them against each other. But which one will gain electrons and become negatively charged, and which one will lose electrons and become positively charged?
During the rubbing process, the atoms from the different substances interact. The substance with atoms with a greater attracts some electrons from the other substance (with less electron affinity) to itself. On separation, the objects will have opposite electrostatic charges.
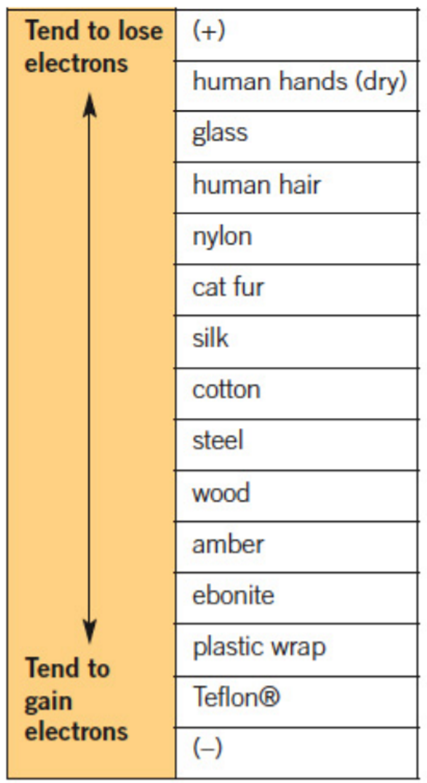
The compares abilities of substances to lose or gain electrons by .
Example 1.1
Use the triboelectric series to determine which object will become positively charged and which will become negatively charged, when wood is rubbed with nylon.
Solution
Step 1: Find the two substances on the table.
Step 2: The substance higher up on the table tends to lose electrons, so it will become positively charged.
Step 3: The substance lower down on the table tends to gain electrons, so it will become negatively charged.
Step 4: By comparison, the answer to this question will be:
- nylon will become positively charged
- wood will become negatively charged.
Activity 1.1: Investigate how objects becomes charged
Time required: 10 minutes
What you need:
- internet access
What to do:
- Open the following link to the Physics Classroom to play ‘Name that charge’.

- Follow the instructions and complete the activity.
What did you find?
Objects can become charged by friction. The direction in which the negative charges move (electrons) is determined by the relative electron affinity between the two substances. Electrons will move from the substance with the lesser electron affinity into the substance with the greater electron affinity.
Exercise 1.1
For the pairs of substances below determine the charge on each when rubbed together and then separated:
- Cat fur and amber
- Ebonite and silk
- Glass and human hair
- Steel and wood
The full solutions are at the end of the unit.
The law of conservation of charge
In all the examples we have looked at charge was not created or destroyed. It moved from one material to another. The total amount of positive charge and the total amount of negative charge in the whole system remained constant. It was merely the redistribution of the negative charges that resulted in one object becoming positively charged and the other becoming negatively charged.
This concept is referred to as the .
Summary
In this unit you have learnt the following:
- There are two kinds of charge: positive and negative.
- Positive charge is carried by protons in the nucleus of atoms.
- Negative charge is carried by electrons.
- Objects become charged because of a change in the number of electrons.
- Friction causes electrons to move from one substance to another.
- Objects can be positively charged, negatively charged or neutral.
- Objects that are neutral have equal numbers of positive and negative charge.
- Charge is neither created nor destroyed, it can only be transferred.
Unit 1: Assessment
Suggested time to complete: 10 minutes
- Name the two kinds of charge and the sub-atomic particles responsible for each?
- Choose the correct answer to the following multiple-choice questions:
- Objects become positively charged when:
- they gain electrons
- they gain protons
- they lose electrons
- they lose protons
- When amber is rubbed with cotton cloth:
- electrons move from the cotton cloth to the amber
- electrons move from the amber to the cotton cloth
- protons move from the cotton cloth to the amber
- protons move from the amber to the cotton cloth
- When human hair is rubbed with silk:
- the human hair becomes positively charged
- the human hair remains neutral
- the human hair becomes negatively charged
- the silk becomes positively charged
- Electrons will be most likely to move from:
- plastic wrap to amber
- steel to ebonite
- glass to Teflon®
- nylon to silk
- Objects become positively charged when:
- Explain what happens when an object becomes charged by friction.
- Given that silicon has a greater electron affinity compared to aluminium, explain:
- how you could charge two objects made of these substances.
- the charge that develops on the aluminium, in terms of the movement of electrons.
- State the law of conservation of charge.
The full solutions are at the end of the unit.
Unit 1: Solutions
Exercise 1.1
- cat fur – positive; amber – negative
(cat fur has a greater tendency to lose electrons compared to amber according to the triboelectric series) - ebonite – negative; silk – positive
(ebonite has a greater tendency to gain electrons compared to silk according to the triboelectric series) - glass – positive; human hair – negative
(glass has a greater tendency to lose electrons compared to human hair according to the triboelectric series) - steel – positive; wood – negative
(steel has a greater tendency to lose electrons compared to wood according to the triboelectric series).
Unit 1: Assessment
- Positive – protons
Negative – electrons - .
- C
- A (use the triboelectric series)
- A (use the triboelectric series)
- C (these two substances exhibit the greatest difference in their electron affinity)
- During the rubbing process, electrons will move from the substance with lesser electron affinity to the substance with greater electron affinity.
- .
- You can rub the two substances together.
- The aluminium will lose electrons and therefore become positively charged.
- The net charge of an isolated system remains constant.
Media Attributions
- img01_Figure1 © Siyavula is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license
- img02_Figure2 © Siyavula is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license
- img03_Figure3 © Siyavula is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license
- img04_Figure4 © Siyavula is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license
- img05_Figure5 © L Kleynhans is licensed under a CC BY (Attribution) license
- QR_Code_PSL2SO42U1_1
- Parts of the text in this unit were sourced from Siyavula Physical Science Gr 10 Learner’s Book, Chapter 16, released under a CC-BY licence ↵
to have a shortage
to have extra
the tendency of a substance to attract electrons from other substances
an ordered list of substances based on their electron affinity
rubbing two surfaces against each other
the net charge of an isolated system remains constant
