Matter and materials: Identify and describe atoms as the basic building block
Unit 1: The atom
Emma Harrage
Unit outcomes
By the end of this unit you will be able to:
- Draw atoms with the protons and neutrons in a nucleus and electrons in orbiting shells
- Identify an atom according to a diagram
Introduction
In this unit you will learn about the atom, which is the basic building block of matter. You will learn about the structure of the atom; how atoms are made up of tiny particles called protons, electrons and neutrons and the position of electrons on shells and the protons and neutrons in the nucleus.
The structure of the atom
An is the basic building block of all matter. Everything you can think of is made of atoms. Atoms are so small that there are approximately 10 million million or [latex]\scriptsize 10^{13}[/latex] of them in a pencil dot.
In work published in 1808, John Dalton proposed that all matter is composed of very small things which he called atoms. Atoms are made up of sub-atomic particles called electrons, neutrons and protons. Electrons were discovered by J.J Thompson in 1897, neutrons in 1932 by James Chadwick, and Ernest Rutherford discovered protons in 1909.
Rutherford’s model of the atom described the atom as a tiny, dense, positively charged core called a nucleus surrounded by lighter, negatively charged electrons.
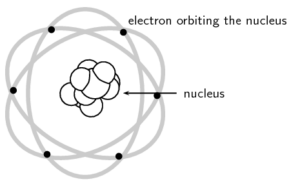
Niels Bohr proposed that the electrons could orbit the nucleus in certain special orbits at different energy levels around the nucleus.
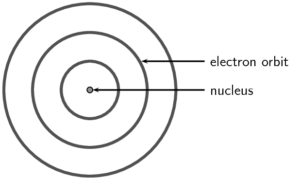
As a result of the work done by previous scientists on atomic models, scientists now have a good idea of what an atom looks like. This knowledge is important because it helps us to understand why materials have different properties and why some materials bond with others.
Atoms consist of three different types of subatomic particles:
- negatively charged electrons
- neutrons which have no charge
- positively charged protons.
The protons and neutrons are found in the nucleus and the electrons orbit around the nucleus in shells. These are called .
The electron
The is a very tiny particle. It has a mass of [latex]\scriptsize 9.11 \times 10^{-31}[/latex] kg. The electron carries one unit of negative electric charge.
The nucleus
Unlike the electron, the nucleus can be broken up into smaller building blocks called protons and neutrons. Together, the protons and neutrons are called .
The proton
Each carries one unit of positive electric charge. Since we know that atoms are electrically neutral, they do not carry any extra charge, then the number of protons in an atom has to be the same as the number of electrons to balance out the positive and negative charge. The total positive charge of a nucleus is equal to the number of protons in the nucleus. A proton is much heavier than an electron (10 000 times heavier!) and has a mass of [latex]\scriptsize 1.6726 \times 10^{-27}[/latex] kg. When we talk about the atomic mass of an atom, we are mostly referring to the combined mass of the protons and neutrons.
The neutron
The is electrically neutral, so it carries no charge at all. Like a proton, it is much heavier than an electron and its mass is [latex]\scriptsize 1.6749 \times 10^{-27}[/latex] kg (slightly heavier than a proton).
Exercise 1.1
- The three basic components of an atom are:
- protons, neutrons, and ions
- protons, neutrons, and electrons
- protons, neutrinos, and ions
- protium, deuterium, and tritium
- The charge of an atom is:
- positive
- neutral
- negative
- none of the above
The full solutions are at the end of the unit.
Activity 1.1: Building atoms
Suggested time: 15 minutes
What you need:
- internet access
What to do:
Open the Build an atom simulation. Click on ‘Build an Atom’, then select ‘Atom’.
Build atoms for different elements by adding protons, neutrons and electrons to the atom diagram.
Summary
In this unit you have learnt the following:
- An atom is made up of a central nucleus (containing protons and neutrons), surrounded by electrons. Most of the atom is empty space.
- Electrons are negatively charged particles which orbit the nucleus in shells.
- Protons are positively charged particles found in the nucleus.
- Neutrons are neutrally charged particles found in the nucleus.
Unit 1: Assessment
Suggested time to complete: 10 minutes
- What is the centre of an atom called?
- The protons
- The nucleus
- The electrons
- What is the charge on an electron?
- One positive charge
- No charge
- One negative charge
- Which of the three sub-atomic particles is the lightest?
- The proton
- The neutron
- The electron
- If atoms contain charged particles, why do they not have a charge?
- They contain the same number of protons as electrons.
- The charge is locked away in the nucleus.
- They contain equal numbers of protons and neutrons.
The full solutions are at the end of the unit.
Unit 1: Solutions
Exercise 1.1
- b
- b
Unit 1: Assessment
- b
- c
- c
- a
Media Attributions
- Rutherfords model of the atom © Siyavula is licensed under a CC BY (Attribution) license
- Bohrs model of the atom © Siyavula is licensed under a CC BY (Attribution) license
simplest form of matter; made of equal numbers of protons, electrons, and neutrons
The place/area where electrons can be found orbiting the nucleus
negatively charged particle which orbits the nucleus
protons and neutrons found in the nucleus
positively charged particle found in the nucleus
neutrally charged particle found in the nucleus
